Gingerenone ACAS# 128700-97-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128700-97-0 | SDF | Download SDF |
| PubChem ID | 5281775 | Appearance | Oil |
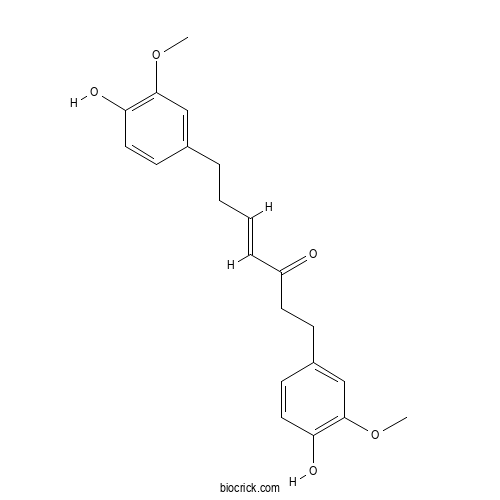
| Formula | C21H24O5 | M.Wt | 356.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hept-4-en-3-one | ||
| SMILES | COC1=C(C=CC(=C1)CCC=CC(=O)CCC2=CC(=C(C=C2)O)OC)O | ||
| Standard InChIKey | FWDXZNKYDTXGOT-GQCTYLIASA-N | ||
| Standard InChI | InChI=1S/C21H24O5/c1-25-20-13-15(8-11-18(20)23)5-3-4-6-17(22)10-7-16-9-12-19(24)21(14-16)26-2/h4,6,8-9,11-14,23-24H,3,5,7,10H2,1-2H3/b6-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Gingerenone A Dilution Calculator

Gingerenone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0292 mL | 28.0584 mL | 56.1167 mL | 70.1459 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6117 mL | 7.0146 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (3S,5S)-[6]-Gingerdiol
Catalog No.:BCN0638
CAS No.:143615-76-3
- 4-(4-Hydroxy-3-methoxyphenyl)butane-1,2-diol
Catalog No.:BCN0637
CAS No.:39115-22-5
- Bisisorhapontigenin B
Catalog No.:BCN0636
CAS No.:
- Isocalophyllic acid
Catalog No.:BCN0635
CAS No.:157810-76-9
- Calophyllic acid
Catalog No.:BCN0634
CAS No.:36626-19-4
- (3S,5S)-[4]-Gingerdiol
Catalog No.:BCN0633
CAS No.:1448789-37-4
- Annphenone
Catalog No.:BCN0632
CAS No.:61775-18-6
- Colelomycerone A
Catalog No.:BCN0631
CAS No.:1191896-73-7
- Campsiketalin
Catalog No.:BCN0630
CAS No.:93675-96-8
- Ethyl 13-hydroxy-α-linolenate
Catalog No.:BCN0629
CAS No.:123435-84-7
- Ethyl 3,5-di-O-caffeoylquinate
Catalog No.:BCN0628
CAS No.:143051-74-5
- 1,6,2',6'-O-Tetraacetyl-3-O-trans-p-coumaroylsucrose
Catalog No.:BCN0627
CAS No.:138195-49-0
- cis-ε-Viniferin
Catalog No.:BCN0640
CAS No.:62250-12-8
- Chiricanine A
Catalog No.:BCN0641
CAS No.:350593-30-5
- Gneafricanin F
Catalog No.:BCN0642
CAS No.:477561-12-9
- Nandinaside A
Catalog No.:BCN0643
CAS No.:1813517-23-5
- [6]-Gingerdiol
Catalog No.:BCN0644
CAS No.:154905-69-8
- [4]-Gingerol
Catalog No.:BCN0645
CAS No.:41743-68-4
- Nujiangefolin A
Catalog No.:BCN0646
CAS No.:1393644-51-3
- 1,7-Bis(3,4-dihydroxyphenyl)heptane-3,5-diyl diacetate
Catalog No.:BCN0647
CAS No.:138870-97-0
- 3''-Demethylhexahydrocurcumin
Catalog No.:BCN0648
CAS No.:881008-71-5
- Cannabisin C
Catalog No.:BCN0649
CAS No.:
- Benzyl β-D-glucopyranoside
Catalog No.:BCN0650
CAS No.:4304-12-5
- Homovanillyl alcohol 4-O-glucoside
Catalog No.:BCN0651
CAS No.:104380-15-6
Inhibition of Escherichia coli ATP synthase by dietary ginger phenolics.[Pubmed:34087308]
Int J Biol Macromol. 2021 Jul 1;182:2130-2143.
For centuries, dietary ginger has been known for its antioxidant, anticancer, and antibacterial properties. In the current study, we examined the link between antibacterial properties of 7 dietary ginger phenolics (DGPs)-Gingerenone A, 6-gingerol, 8-gingerol, 10-gingerol, paradol, 6-shogaol, and zingerone-and inhibition of bacterial ATP synthase. DGPs caused complete (100%) inhibition of wild-type Escherichia coli membrane-bound F1Fo ATP synthase, but partial and variable (0%-87%) inhibition of phytochemical binding site mutant enzymes alphaR283D, alphaE284R, betaV265Q, and gammaT273A. The mutant enzyme ATPase activity was 16-fold to 100-fold lower than that of the wild-type enzyme. The growth of wild-type, null, and mutant strains in the presence of the 7 DGPs were abrogated to variable degrees on limiting glucose and succinate media. DGPs-caused variable inhibitory profiles of wild-type and mutant ATP synthase confirm that residues of alpha-, beta-, and gamma-subunits are involved in the formation of phytochemical binding site. The variable degree of growth in the presence of DGPs also indicates the possibility of molecular targets other than ATP synthase. Our results establish that antibacterial properties of DGPs can be linked to the binding and inhibition of bacterial ATP synthase. Therefore, bacterial ATP synthase is a valuable molecular target for DGPs.
In Vitro and In Vivo Antiviral Activity of Gingerenone A on Influenza A Virus Is Mediated by Targeting Janus Kinase 2.[Pubmed:33050000]
Viruses. 2020 Oct 8;12(10). pii: v12101141.
Janus kinase (JAK) inhibitors have been developed as novel immunomodulatory drugs and primarily used for treating rheumatoid arthritis and other inflammatory diseases. Recent studies have suggested that this category of anti-inflammatory drugs could be potentially useful for the control of inflammation "storms" in respiratory virus infections. In addition to their role in regulating immune cell functions, JAK1 and JAK2 have been recently identified as crucial cellular factors involved in influenza A virus (IAV) replication and could be potentially targeted for antiviral therapy. Gingerenone A (Gin A) is a compound derived from ginger roots and a dual inhibitor of JAK2 and p70 S6 kinase (S6K1). Our present study aimed to determine the antiviral activity of Gin A on influenza A virus (IAV) and to understand its mechanisms of action. Here, we reported that Gin A suppressed the replication of three IAV subtypes (H1N1, H5N1, H9N2) in four cell lines. IAV replication was also inhibited by Ruxolitinib (Rux), a JAK inhibitor, but not by PF-4708671, an S6K1 inhibitor. JAK2 overexpression enhanced H5N1 virus replication and attenuated Gin A-mediated antiviral activity. In vivo experiments revealed that Gin A treatment suppressed IAV replication in the lungs of H5N1 virus-infected mice, alleviated their body weight loss, and prolonged their survival. Our study suggests that Gin A restricts IAV replication by inhibiting JAK2 activity; Gin A could be potentially useful for the control of influenza virus infections.
Gingerenone A Sensitizes the Insulin Receptor and Increases Glucose Uptake by Inhibiting the Activity of p70 S6 Kinase.[Pubmed:30296358]
Mol Nutr Food Res. 2018 Dec;62(23):e1800709.
SCOPE: The bioactive constituents in ginger extract are responsible for anti-hyperglycemic effects and the underlying mechanisms are incompletely understood. Gingerenone A (Gin A) has been identified as an inhibitor of p70 S6 (S6K1), a kinase that plays a critical role in the pathogenesis of insulin resistance. This study aims to evaluate if Gin A can sensitize the insulin receptor by inhibiting S6K1 activity. METHODS AND RESULTS: Western blot analysis reveals that Gin A induces phosphatidylinositide-3 kinase (PI3K) feedback activation in murine 3T3-L1 adipocytes and rat L6 myotubes, as evidenced by increased AKT(S473) and S6K1(T389) but decreases S6(S235/236) and insulin receptor substrate 1 (IRS-1)(S1101) phosphorylation. Western blot and immunoprecipitation analysis reveal that Gin A increases insulin receptor tyrosine phosphorylation in L6 myotubes and IRS-1 binding to the PI3K in 3T3-L1 adipocytes. Confocal microscopy reveals that Gin A enhances insulin-induced translocation of glucose transporter 4 (GLUT4) into the cell membrane in L6 cells. 2-NBDG (2-N-(Nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose) Fluorescent assay reveals that Gin A enhances insulin-stimulated glucose uptake in 3T3-L1 adipocytes and L6 myotubes. CONCLUSIONS: Gin A overcomes insulin resistance and increases glucose uptake by inhibiting S6K1 activity. Gin A or other plant-derived S6K1 inhibitors could be developed as novel antidiabetic agents.
Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet-fed mice.[Pubmed:28556482]
Mol Nutr Food Res. 2017 Oct;61(10).
SCOPE: Ginger exerts protective effects on obesity and its complications. Our objectives here are to identify bioactive compounds that inhibit adipogenesis and lipid accumulation in vitro, elucidate the anti-obesity effect of Gingerenone A (GA) in diet-induced obesity (DIO), and investigate whether GA affects adipose tissue inflammation (ATI). METHODS AND RESULTS: Oil red O staining showed that GA had the most potent inhibitory effect on adipogenesis and lipid accumulation in 3T3-L1 cells among ginger components tested at a single concentration (40 muM). Consistent with in vitro data, GA attenuates DIO by reducing fat mass in mice. This was accompanied by a modulation of fatty acid metabolism via activation of AMP-activated protein kinase (AMPK) in vitro and in vivo. Additionally, GA suppressed ATI by inhibiting macrophage recruitment and downregulating pro-inflammatory cytokines. CONCLUSION: These results suggest that GA may be used as a potential therapeutic candidate for the treatment of obesity and its complications by suppressing adipose expansion and inflammation.
Gingerenone A Attenuates Monocyte-Endothelial Adhesion via Suppression of I Kappa B Kinase Phosphorylation.[Pubmed:28513976]
J Cell Biochem. 2018 Jan;119(1):260-268.
During the early stages of atherosclerosis, monocytes bind and migrate into the endothelial layer, promoting inflammation within the aorta. In order to prevent the development of atherosclerosis, it is critical to inhibit such inflammation. The therapeutic effects of ginger have been investigated in several models of cardiovascular disease. However, although a number of previous studies have focused on specific compounds, the mechanisms of action responsible remain unclear. Here, we investigated five major compounds present in ginger, and observed that Gingerenone A exhibited the strongest inhibitory effects against tumor necrosis factor (TNF)-alpha and lipopolysaccharide (LPS) induced monocyte-endothelial adhesion. Furthermore, Gingerenone A significantly suppressed the expression of TNF-alpha and LPS-induced vascular cell adhesion molecule-1 (VCAM-1) and chemokine (C-C motif) ligand 2 (CCL2), key mediators of the interaction between monocytes, and endothelial cells. Transactivation of nuclear factor-kappaB (NF-kappaB), which is a key transcription factor of VCAM-1 and CCL2, was induced by TNF-alpha and LPS, and inhibited by treatment of Gingerenone A. Gingerenone A also inhibited the phosphorylation of NF-kappaB inhibitor (IkappaB) alpha and IkappaB Kinase. Taken together, these results demonstrate that Gingerenone A attenuates TNF-alpha and LPS-induced monocyte adhesion and the expression of adhesion factors in endothelial cells via the suppression of NF-kappaB signaling. J. Cell. Biochem. 119: 260-268, 2018. (c) 2017 Wiley Periodicals, Inc.
MS-Based Metabolite Profiling of Aboveground and Root Components of Zingiber mioga and Officinale.[Pubmed:26404226]
Molecules. 2015 Sep 3;20(9):16170-85.
Zingiber species are members of the Zingiberaceae family, and are widely used for medicinal and food purposes. In this study aboveground and root parts of Zingiber mioga and Zingiber officinale were subjected to metabolite profiling by ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF-MS) and gas chromatography time-of-flight mass spectrometry (GC-TOF-MS) in order to characterize them by species and parts and also to measure bioactivities. Both primary and secondary metabolites showed clear discrimination in the PCA score plot and PLS-DA by species and parts. Tetrahydrocurcumin, diarylheptanoid, 8-gingerol, and 8-paradol were discriminating metabolites between Z. mioga and Z. officinale that were present in different quantities. Eleven flavonoids, six amino acids, six organic acids, four fatty acids, and Gingerenone A were higher in the aboveground parts than the root parts. Antioxidant activities were measured and were highest in the root part of Z. officinale. The relatively high contents of tetrahydrocurcumin, diarylheptanoid, and galanganol C in the root part of Z. officinale showed highly positive correlation with bioactivities based on correlation assay. On the basis of these results, we can suggest different usages of structurally different parts of Zingiber species as food plants.
Identification of a Dual Inhibitor of Janus Kinase 2 (JAK2) and p70 Ribosomal S6 Kinase1 (S6K1) Pathways.[Pubmed:26242912]
J Biol Chem. 2015 Sep 25;290(39):23553-62.
Bioactive phytochemicals can suppress the growth of malignant cells, and investigation of the mechanisms responsible can assist in the identification of novel therapeutic strategies for cancer therapy. Ginger has been reported to exhibit potent anti-cancer effects, although previous reports have often focused on a narrow range of specific compounds. Through a direct comparison of various ginger compounds, we determined that Gingerenone A selectively kills cancer cells while exhibiting minimal toxicity toward normal cells. Kinase array screening revealed JAK2 and S6K1 as the molecular targets primarily responsible for Gingerenone A-induced cancer cell death. The effect of Gingerenone A was strongly associated with relative phosphorylation levels of JAK2 and S6K1, and administration of Gingerenone A significantly suppressed tumor growth in vivo. More importantly, the combined inhibition of JAK2 and S6K1 by commercial inhibitors selectively induced apoptosis in cancer cells, whereas treatment with either agent alone did not. These findings provide rationale for dual targeting of JAK2 and S6K1 in cancer for a combinatorial therapeutic approach.
Anthelmintic constituents from ginger (Zingiber officinale) against Hymenolepis nana.[Pubmed:25063389]
Acta Trop. 2014 Dec;140:50-60.
This study investigated the anthelmintic activity of Gingerenone A, [6]-dehydrogingerdione, [4]-shogaol, 5-hydroxy-[6]-gingerol, [6]-shogaol, [6]-gingerol, [10]-shogaol, [10]-gingerol, hexahydrocurcumin, 3R,5S-[6]-gingerdiol and 3S,5S-[6]-gingerdiol, a constituent isolate from the roots of ginger, for the parasite Hymenolepis nana. The cestocidal activity or ability to halt spontaneous parasite movement (oscillation/peristalsis) in H. nana of above constituents was reached from 24 to 72h in a time- and dose-dependent manner, respectively. The [10]-shogaol and [10]-gingero1 have maximum lethal efficacy and loss of spontaneous movement than the others at 24-72h. In addition, worms treated with 1 and 10muM [10]-gingero1, more than 30% had spontaneous movement of oscillation at 72h but [10]-shogaol at 72h only about 15-20% of oscillation. This showing that [10]-gingero1 had less loss of spontaneous movement efficacy than [10]-shogaol. After exposure to 200muM [10]-shogaol, 100% of H. nana had died at 12h rather than died at 24h for [10]-gingerol, showing that [10]-gingero1 had less lethal efficacy than [10]-shogaol. In addition, these constituents of ginger showed effects against peroxyl radical under cestocidal activity. In order to evaluate the cestocidal activity and cytokine production caused by ginger's extract R0 in the H. nana infected mice, we carried out in vivo examination about H. nana infected mice BALB/c mice were inoculated orally with 500 eggs. After post-inoculation, R0 (1g/kg/day) was administered orally for 10 days. The R0 exhibited cestocidal activity in vivo of significantly reduced worms number and cytokines production by in vitro Con A-stimulated spleen cells showed that INF-gamma and IL-2 were significantly increases by R0. IL-4, IL-5, IL-6, IL-10 and IL-13 were significantly decreases and Murine KC and IL-12 were not significantly changes by R0. Together, these findings first suggest that these constituents of ginger might be used as cestocidal agents against H. nana.
[Phenolic and amide constituents from Lycianthes marlipoensis].[Pubmed:22256755]
Zhongguo Zhong Yao Za Zhi. 2011 Sep;36(18):2507-10.
Ten known phenolic compounds including [4]-gingerol (1), [6]-gingerol (2), [10]-gingerol (3), (3S,5S)-3,5-dihydroxy-1-(4-hydroxy-3-methoxyphenyl) decane (4), (3R,5S) -3, 5-dihydroxy-1-(4-hydroxy-3-methoxyphenyl) decane (5), [6]-shogaol (6), [10]-shogaol (7), Gingerenone A (8), hexahydrocurcumin (9), and (3R,5R)-3,5-dihydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl) heptane (10), and seven amides including piperine (11), isochavicine (12), isopiperine (13), N-trans-p-coumaroyl tyramine (14), N-trans-feruloyl tyramine (15), N-trans-p-coumaroyl octopamine (16), N-trans-feruloyl octopamine (17), were isolated and identified from the roots of Lycianthes marlipoensis. Compounds 1-13 and 17 were isolated from the genus Lycianthes for the first time.
Modulation of macrophage functions by compounds isolated from Zingiber officinale.[Pubmed:19031369]
Planta Med. 2009 Feb;75(2):148-51.
Bioactivity-guided fractionation of Zingiber Officinale (zingiberaceae) led us to isolate 14 compounds, -gingerol ( 1), -gingerol ( 2), -gingerol ( 3), -gingerol ( 4), -paradol ( 5), -shogaol ( 6), -shogaol ( 7), 1-dehydro- -gingerdione ( 8), -gingerdione ( 9), hexahydrocurcumin ( 10), tetrahydrocurcumin ( 11), Gingerenone A ( 12), 1,7-bis-(4' hydroxyl-3' methoxyphenyl)-5-methoxyhepthan-3-one ( 13), and methoxy- -gingerol ( 14). Using the RAW 264.7 cell line, the inhibitory effects on nitric oxide production induced by lipopolysaccharide and the stimulatory effects on phagocytosis of these compounds were evaluated. Compounds 7, 8, and 9 significantly decreased lipopolysaccharide-induced nitric oxide production, and compounds 7 and 8 significantly reduced inducible nitric oxide synthase expression. Among them, compound 8 also showed significant stimulatory effects on phagocytosis.


