CylindramideCAS# 147362-39-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147362-39-8 | SDF | Download SDF |
| PubChem ID | 132350824 | Appearance | Powder |
| Formula | C27H34N2O5 | M.Wt | 466.57 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
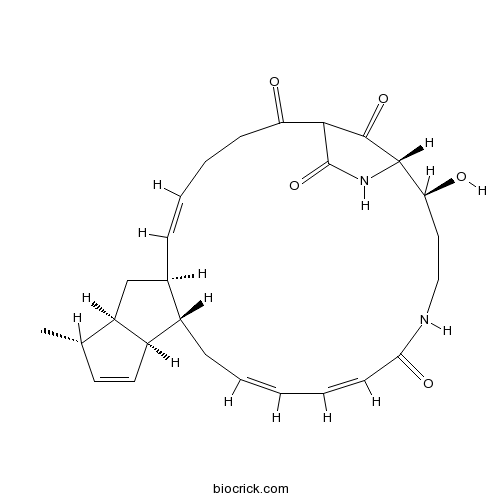
| Chemical Name | (5E,7S,9R,10S,13S,14S,16Z,18E,24S,25S)-24-hydroxy-10-methyl-21,26-diazatetracyclo[23.2.1.07,14.09,13]octacosa-5,11,16,18-tetraene-2,20,27,28-tetrone | ||
| SMILES | CC1C=CC2C1CC3C2CC=CC=CC(=O)NCCC(C4C(=O)C(C(=O)CCC=C3)C(=O)N4)O | ||
| Standard InChIKey | NTIRDRDLRPRTGP-GTNGLYRXSA-N | ||
| Standard InChI | InChI=1S/C27H34N2O5/c1-16-11-12-19-18-8-3-2-4-10-23(32)28-14-13-22(31)25-26(33)24(27(34)29-25)21(30)9-6-5-7-17(18)15-20(16)19/h2-5,7,10-12,16-20,22,24-25,31H,6,8-9,13-15H2,1H3,(H,28,32)(H,29,34)/b3-2-,7-5+,10-4+/t16-,17+,18-,19+,20+,22-,24?,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cylindramide,a cytotoxic tetramic acid lactam isolated from the marine sponge Halichondria cylindrata, it is cytotoxic against B16 melanoma cells with an IC50 of 0.8 ug/mL. 2. Cylindramide shows promising antiproliferative activity against several tumour cell lines. 3. (+)-Cylindramide has anti-microbial properties. |
| Targets | Antifection |

Cylindramide Dilution Calculator

Cylindramide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1433 mL | 10.7165 mL | 21.433 mL | 42.866 mL | 53.5825 mL |
| 5 mM | 0.4287 mL | 2.1433 mL | 4.2866 mL | 8.5732 mL | 10.7165 mL |
| 10 mM | 0.2143 mL | 1.0717 mL | 2.1433 mL | 4.2866 mL | 5.3583 mL |
| 50 mM | 0.0429 mL | 0.2143 mL | 0.4287 mL | 0.8573 mL | 1.0717 mL |
| 100 mM | 0.0214 mL | 0.1072 mL | 0.2143 mL | 0.4287 mL | 0.5358 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Antibiotic PF 1052
Catalog No.:BCN1828
CAS No.:147317-15-5
- KRCA 0008
Catalog No.:BCC8007
CAS No.:1472795-20-2
- 7ACC2
Catalog No.:BCC5554
CAS No.:1472624-85-3
- glatiramer acetate
Catalog No.:BCC5642
CAS No.:147245-92-9
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- TT 232
Catalog No.:BCC6248
CAS No.:147159-51-1
- 4-Chloro-L-phenylalanine Hydrochloride
Catalog No.:BCC2638
CAS No.:123053-23-6
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl
Catalog No.:BCC8651
CAS No.:147118-37-4
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
Catalog No.:BCC9031
CAS No.:147118-35-2
- MKT 077
Catalog No.:BCC6241
CAS No.:147366-41-4
- Ligupurpuroside A
Catalog No.:BCC8198
CAS No.:147396-01-8
- Ligupurpuroside B
Catalog No.:BCC8199
CAS No.:147396-02-9
- Azilsartan
Catalog No.:BCC5014
CAS No.:147403-03-0
- Omaveloxolone (RTA-408)
Catalog No.:BCC5281
CAS No.:1474034-05-3
- Telenzepine dihydrochloride
Catalog No.:BCC6946
CAS No.:147416-96-4
- Ginsenoside Rg6
Catalog No.:BCN2706
CAS No.:147419-93-0
- Pitavastatin
Catalog No.:BCC4140
CAS No.:147511-69-1
- Thunberginol C
Catalog No.:BCN1654
CAS No.:147517-06-4
- LY 288513
Catalog No.:BCC5772
CAS No.:147523-65-7
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- Bosentan
Catalog No.:BCC4640
CAS No.:147536-97-8
Total synthesis and NMR investigations of cylindramide.[Pubmed:16389623]
Chemistry. 2006 Mar 8;12(9):2488-503.
Cylindramide (1) was built up from three components: a hydroxyornithine derivative 7, a tetrazolylsulfone 8, and a substituted pentalene subunit 9. Derivative 7 was prepared in a six-step reaction sequence involving the Wittig reaction and a Sharpless asymmetric dihydroxylation starting from N-Boc-3-aminopropanal (12). Tetrazolylsulfone 8 was accessible in four steps from dioxinone 22. The synthesis of the pentalene fragment 9 started from cycloocta-1,5-diene 26, that was converted into enantiopure bicyclo[3.3.0]octanedione 29. The latter was functionalized to give derivative 9. The total synthesis was accomplished by inducing C-C bond formation by Sonogashira coupling of derivatives 9 and 7 followed by olefination with tetrazolylsulfone 8 under Julia-Kocienski conditions, macrocyclization, and subsequent Lacey-Dieckmann condensation to form the tetramic acid unit. As indicated by extensive 1H and 13C NMR spectroscopic investigations (DQF-COSY, ROESY spectra), the stereochemistry of synthetic Cylindramide (1) corresponds with that of the naturally occurring product. ROE data were used for molecular modeling of the lowest-energy structures for Cylindramide.
Synthesis and biological properties of cylindramide derivatives: evidence for calcium-dependent cytotoxicity of tetramic acid lactams.[Pubmed:18798209]
Chembiochem. 2008 Oct 13;9(15):2474-86.
To gain insight into the biological properties of tetramic acid lactam Cylindramide 1, the analogues 4 a-d bearing a cyclopentane ring instead of the pentalene unit were prepared by tandem conjugate addition/enolate trapping of cyclopentenone 10; a Sonogashira or Stille coupling, followed by a Julia-Kocienski olefination, macrolactamisation and Lacey-Dieckmann cyclisation were the key steps. The previous NMR structure of Cylindramide 1, which was based on NOE and J coupling restraints, could be refined by including residual dipolar coupling data measured for a sample of Cylindramide that was aligned in polyacrylonitrile (18 %). Biological screening of Cylindramide 1 and its analogues 2-epi-1, 20 and 4 revealed promising antiproliferative activity against several tumour cell lines. It turned out that the activity is strongly correlated to the functionalised pentalene system. The configuration of the cyclopentane ring and an intact tetramic acid lactam with the correct configuration seem to play an equal role in the cytotoxicity. The antiproliferative activity was found to be calcium dependent. Phenotypic characterisation of the mode of action showed vacuolisation and vesicle formation in the endoplasmic reticulum.
Total synthesis of (+)-cylindramide A.[Pubmed:16433523]
J Am Chem Soc. 2006 Feb 1;128(4):1094-5.
A total synthesis of Cylindramide A has been completed in 19 steps. The key step of the synthesis is a tandem ring-opening-ring-closing-cross metathesis that converts a readily available norbornene into an advanced intermediate.


