glatiramer acetateimmunomodulator CAS# 147245-92-9 |
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- CPI-613
Catalog No.:BCC2287
CAS No.:95809-78-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147245-92-9 | SDF | Download SDF |
| PubChem ID | 3081884 | Appearance | Powder |
| Formula | C25H45N5O13 | M.Wt | 623.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
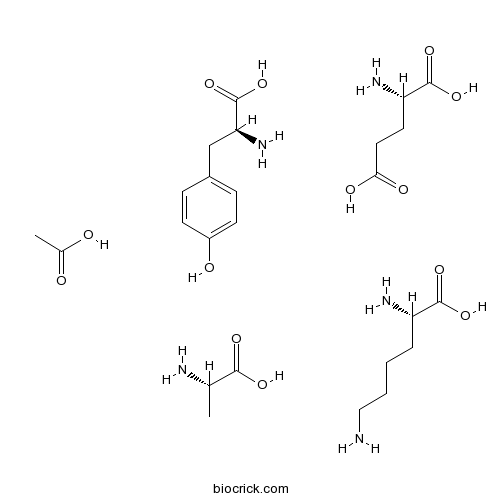
| Chemical Name | acetic acid;(2S)-2-amino-3-(4-hydroxyphenyl)propanoic acid;(2S)-2-aminopentanedioic acid;(2S)-2-aminopropanoic acid;(2S)-2,6-diaminohexanoic acid | ||
| SMILES | CC(C(=O)O)N.CC(=O)O.C1=CC(=CC=C1CC(C(=O)O)N)O.C(CCN)CC(C(=O)O)N.C(CC(=O)O)C(C(=O)O)N | ||
| Standard InChIKey | FHEAIOHRHQGZPC-KIWGSFCNSA-N | ||
| Standard InChI | InChI=1S/C9H11NO3.C6H14N2O2.C5H9NO4.C3H7NO2.C2H4O2/c10-8(9(12)13)5-6-1-3-7(11)4-2-6;7-4-2-1-3-5(8)6(9)10;6-3(5(9)10)1-2-4(7)8;1-2(4)3(5)6;1-2(3)4/h1-4,8,11H,5,10H2,(H,12,13);5H,1-4,7-8H2,(H,9,10);3H,1-2,6H2,(H,7,8)(H,9,10);2H,4H2,1H3,(H,5,6);1H3,(H,3,4)/t8-;5-;3-;2-;/m0000./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

glatiramer acetate Dilution Calculator

glatiramer acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6035 mL | 8.0173 mL | 16.0346 mL | 32.0693 mL | 40.0866 mL |
| 5 mM | 0.3207 mL | 1.6035 mL | 3.2069 mL | 6.4139 mL | 8.0173 mL |
| 10 mM | 0.1603 mL | 0.8017 mL | 1.6035 mL | 3.2069 mL | 4.0087 mL |
| 50 mM | 0.0321 mL | 0.1603 mL | 0.3207 mL | 0.6414 mL | 0.8017 mL |
| 100 mM | 0.016 mL | 0.0802 mL | 0.1603 mL | 0.3207 mL | 0.4009 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
glatiramer acetate
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- TT 232
Catalog No.:BCC6248
CAS No.:147159-51-1
- 4-Chloro-L-phenylalanine Hydrochloride
Catalog No.:BCC2638
CAS No.:123053-23-6
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl
Catalog No.:BCC8651
CAS No.:147118-37-4
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
Catalog No.:BCC9031
CAS No.:147118-35-2
- Maropitant
Catalog No.:BCC1728
CAS No.:147116-67-4
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- 5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one
Catalog No.:BCC8722
CAS No.:147086-79-1
- Alcaftadine
Catalog No.:BCC5260
CAS No.:147084-10-4
- 7ACC2
Catalog No.:BCC5554
CAS No.:1472624-85-3
- KRCA 0008
Catalog No.:BCC8007
CAS No.:1472795-20-2
- Antibiotic PF 1052
Catalog No.:BCN1828
CAS No.:147317-15-5
- Cylindramide
Catalog No.:BCN1832
CAS No.:147362-39-8
- MKT 077
Catalog No.:BCC6241
CAS No.:147366-41-4
- Ligupurpuroside A
Catalog No.:BCC8198
CAS No.:147396-01-8
- Ligupurpuroside B
Catalog No.:BCC8199
CAS No.:147396-02-9
- Azilsartan
Catalog No.:BCC5014
CAS No.:147403-03-0
- Omaveloxolone (RTA-408)
Catalog No.:BCC5281
CAS No.:1474034-05-3
- Telenzepine dihydrochloride
Catalog No.:BCC6946
CAS No.:147416-96-4
- Ginsenoside Rg6
Catalog No.:BCN2706
CAS No.:147419-93-0
- Pitavastatin
Catalog No.:BCC4140
CAS No.:147511-69-1
A Potential Life-Threatening Reaction to Glatiramer Acetate in Rett Syndrome.[Pubmed:28254244]
Pediatr Neurol. 2017 Mar;68:40-43.
BACKGROUND: Rett syndrome is an X-linked dominant neurodevelopmental disorder manifesting with severe intellectual disability in females caused by various mutations in the MECP2 gene. Brain-derived neurotrophic factor (BDNF) is one of the main proteins regulated by the MECP2 protein; its overexpression in the MeCP2 mouse model partially corrects the Rett phenotype. Pharmacologic manipulations that lead to increased BDNF in individuals with Rett syndrome are expected to have a positive effect on the disorder. glatiramer acetate, a well-known and safe multiple sclerosis immune modulator, increases BDNF levels in multiple sclerosis animal models and patients responding to treatment, as well as in Rett mouse models. METHODS: Fourteen patients with mutation-proven Rett syndrome were recruited for a clinical trial with glatiramer acetate. Baseline data and follow-up data were collected during the trial, which had to be stopped because of a severe adverse event. Our objective is to describe this unexpected potentially life-threatening event in response to glatiramer in patients with Rett syndrome. RESULTS: Four of 14 patients with Rett syndrome who were recruited and treated with daily injections of glatiramer acetate as part of an open-label clinical trial developed an exaggerated immediate postinjection response, which was experienced as life threatening in three of the patients, necessitating arrest of the trial. CONCLUSION: Despite the known safety profile of glatiramer acetate in adult and pediatric patients with multiple sclerosis, its use in Rett syndrome should be cautiously reconsidered. The described severe adverse event can be related to these patients' primary autonomic nervous system dysfunction.
Demonstration of Biological and Immunological Equivalence of a Generic Glatiramer Acetate.[Pubmed:28240190]
CNS Neurol Disord Drug Targets. 2017;16(6):714-723.
BACKGROUND: In April 2015, the US Food and Drug Administration approved the first generic glatiramer acetate, Glatopa(R) (M356), as fully substitutable for Copaxone(R) 20 mg/mL for relapsing forms of multiple sclerosis (MS). This approval was accomplished through an Abbreviated New Drug Application that demonstrated equivalence to Copaxone. METHOD: This article will provide an overview of the methods used to establish the biological and immunological equivalence of the two glatiramer acetate products, including methods evaluating antigenpresenting cell (APC) biology, T-cell biology, and other immunomodulatory effects. RESULTS: In vitro and in vivo experiments from multiple redundant orthogonal assays within four biological processes (aggregate biology, APC biology, T-cell biology, and B-cell biology) modulated by glatiramer acetate in MS established the biological and immunological equivalence of Glatopa and Copaxone and are described. The following were observed when comparing Glatopa and Copaxone in these experiments: equivalent delays in symptom onset and reductions in "disease" intensity in experimental autoimmune encephalomyelitis; equivalent dose-dependent increases in Glatopa- and Copaxone- induced monokine-induced interferon-gamma release from THP-1 cells; a shift to a T helper 2 phenotype resulting in the secretion of interleukin (IL)-4 and downregulation of IL-17 release; no differences in immunogenicity and the presence of equivalent "immunofingerprints" between both versions of glatiramer acetate; and no stimulation of histamine release with either glatiramer acetate in basophilic leukemia 2H3 cell lines. CONCLUSION: In summary, this comprehensive approach across different biological and immunological pathways modulated by glatiramer acetate consistently supported the biological and immunological equivalence of Glatopa and Copaxone.
Glatiramer acetate treatment persistence - but not adherence - in multiple sclerosis patients is predicted by health-related quality of life and self-efficacy: a prospective web-based patient-centred study (CAIR study).[Pubmed:28292329]
Health Qual Life Outcomes. 2017 Mar 14;15(1):50.
BACKGROUND: In patients with relapsing remitting multiple sclerosis (RRMS) the persistence of and adherence to disease modifying drug (DMD) treatment is inadequate. To take individualised measures there is a need to identify patients with a high risk of non-persistence or non-adherence. As patient-related factors have a major influence on persistence and adherence, we investigated whether health-related quality of life (HRQoL) and self-efficacy could predict persistence or adherence. METHODS: In a prospective web-based patient-centred study in 203 RRMS patients, starting treatment with glatiramer acatete (GA) 20 mg subcutaneously daily, we measured physical and mental HRQoL (Multiple Sclerosis Quality of Life-54 questionnaire), functional and control self-efficacy (Multiple Sclerosis Self-Efficacy Scale), the 12-month persistence rate and, in persistent patients, the percentage of missed doses. HRQoL and self-efficacy were compared between persistent and non-persistent patients, and between adherent and non-adherent patients. Logistic regression analysis was used to assess whether persistence and adherence were explained by HRQoL and self-efficacy. RESULTS: Persistent patients had higher baseline physical (mean 58.1 [standard deviation, SD] 16.9) and mental HRQoL (63.8 [16.8]) than non-persistent patients (49.5 [17.6]; 55.9 [20.4]) (P = 0.001; P = 0.003) with no differences between adherent and non-adherent patients (P = 0.46; P = 0.54). Likewise, in persistent patients function (752 [156]) and control self-efficacy (568 [178]) were higher than in non-persistent patients (689 [173]; 491 [192]) (P = 0.009; P = 0.004), but not in adherent vs. non-adherent patients (P = 0.26; P = 0.82). Logistic regression modelling identified physical HRQoL and control self-efficacy as factors that explained persistence. Based on predicted scores from the model, patients were classified into quartiles and the percentage of non-persistent patients per quartile was calculated: non-persistence in the highest quartile was 23.4 vs. 53.2% in the lowest quartile. Risk differentiation with respect to adherence was not possible. Based on these findings we propose a practical work-up scheme to identify patients with a high risk of non-persistence and to identify persistence-related factors. CONCLUSIONS: Findings suggest that pre-treatment physical HRQoL and control self-efficacy may identify RRMS patients with a high risk of early discontinuation of injectable DMD treatment. Targeting of high-risk patients may enable the efficient use of persistence-promoting measures. TRIAL REGISTRATION: Nederlands Trial Register code: NTR2432 .
Comparative effectiveness of delayed-release dimethyl fumarate versus glatiramer acetate in multiple sclerosis patients: results of a matching-adjusted indirect comparison.[Pubmed:28350241]
J Comp Eff Res. 2017 Jun;6(4):313-323.
AIM: Using matching-adjusted indirect comparison, we compared efficacy outcomes in patients with relapsing-remitting multiple sclerosis treated with delayed-release dimethyl fumarate (DMF) or glatiramer acetate (GA). MATERIALS & METHODS: An indirect comparison of DMF (patient-level data) and GA (aggregate data) was conducted, with average baseline characteristics of DMF patients weighted to match those for GA patients. Direct comparison of DMF and GA was conducted in CONFIRM. Final results pooled the indirect and direct comparisons using meta-analysis. RESULTS: After matching, baseline characteristics were balanced between DMF and GA patients. Compared with GA, efficacy was significantly in favor of DMF as measured by annualized relapse rate (rate ratio: 0.76; 95% CI: 0.57-1.00; p = 0.0474) and 12-week confirmed disability progression (risk ratio: 0.59; 95% CI: 0.46-0.76; p < 0.0001). CONCLUSION: DMF demonstrated superior clinical efficacy versus GA.


