Mycophenolate MofetilIMPDH inhibitor CAS# 128794-94-5 |
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- CPI-613
Catalog No.:BCC2287
CAS No.:95809-78-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128794-94-5 | SDF | Download SDF |
| PubChem ID | 5281078 | Appearance | Powder |
| Formula | C23H31NO7 | M.Wt | 433.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RS 61443; TM-MMF | ||
| Solubility | DMSO : 100 mg/mL (230.69 mM; Need ultrasonic) | ||
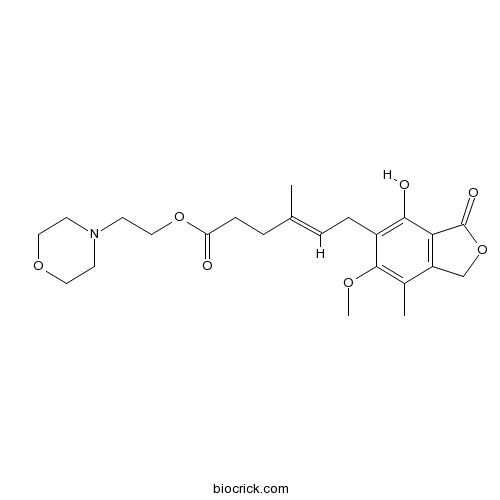
| Chemical Name | 2-morpholin-4-ylethyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-2-benzofuran-5-yl)-4-methylhex-4-enoate | ||
| SMILES | CC1=C(C(=C(C2=C1COC2=O)O)CC=C(C)CCC(=O)OCCN3CCOCC3)OC | ||
| Standard InChIKey | RTGDFNSFWBGLEC-SYZQJQIISA-N | ||
| Standard InChI | InChI=1S/C23H31NO7/c1-15(5-7-19(25)30-13-10-24-8-11-29-12-9-24)4-6-17-21(26)20-18(14-31-23(20)27)16(2)22(17)28-3/h4,26H,5-14H2,1-3H3/b15-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Immunosuppressant prodrug of mycophenolic acid (MPA). Hydrolyzed by esterases to release MPA (a reversible inhibitor of inosine monophosphate dehydrogenase). Used to prevent organ rejection after transplantation. Displays inhibitory activity of lymphocyte functions. Inhibits type 1 collagen expression, enhances MMP-1 expression, and reduces α-smooth muscle actin gene expression. |

Mycophenolate Mofetil Dilution Calculator

Mycophenolate Mofetil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3069 mL | 11.5343 mL | 23.0686 mL | 46.1372 mL | 57.6715 mL |
| 5 mM | 0.4614 mL | 2.3069 mL | 4.6137 mL | 9.2274 mL | 11.5343 mL |
| 10 mM | 0.2307 mL | 1.1534 mL | 2.3069 mL | 4.6137 mL | 5.7671 mL |
| 50 mM | 0.0461 mL | 0.2307 mL | 0.4614 mL | 0.9227 mL | 1.1534 mL |
| 100 mM | 0.0231 mL | 0.1153 mL | 0.2307 mL | 0.4614 mL | 0.5767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mycophenolate Mofetil is a non-competitive, selective and reversible inhibitor of inosine monophosphate dehydrogenase I/II with IC50 of 39 nM and 27 nM, respectively.
- Kalopanaxsaponin H
Catalog No.:BCN2572
CAS No.:128730-82-5
- Eucamalduside A
Catalog No.:BCN7321
CAS No.:1287220-29-4
- FRAX597
Catalog No.:BCC4172
CAS No.:1286739-19-2
- 1,6-O,O-Diacetylbritannilactone
Catalog No.:BCN7792
CAS No.:1286694-67-4
- PyBOP
Catalog No.:BCC2820
CAS No.:128625-52-5
- Z(2-Br)-Osu
Catalog No.:BCC2806
CAS No.:128611-93-8
- Ospemifene
Catalog No.:BCC5557
CAS No.:128607-22-7
- Pingpeimine C
Catalog No.:BCN8411
CAS No.:128585-96-6
- ML167
Catalog No.:BCC5348
CAS No.:1285702-20-6
- GSK2578215A
Catalog No.:BCC6243
CAS No.:1285515-21-0
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
- Ophiogenin-3-O-alpha-L-rhaMnopyranosyl-(1→2)-beta-D-glucopyranoside
Catalog No.:BCN1587
CAS No.:128502-94-3
- Fmoc-D-Asp(OtBu)-OH
Catalog No.:BCC3471
CAS No.:12883-39-3
- Fargesol
Catalog No.:BCN6421
CAS No.:128855-64-1
- Maohuoside A
Catalog No.:BCN5348
CAS No.:128988-55-6
- Suramin hexasodium salt
Catalog No.:BCC7079
CAS No.:129-46-4
- Methysergide maleate
Catalog No.:BCC5698
CAS No.:129-49-7
- SP 600125
Catalog No.:BCC2474
CAS No.:129-56-6
- Buclizine HCl
Catalog No.:BCC4516
CAS No.:129-74-8
- Rivastigmine Tartrate
Catalog No.:BCC3851
CAS No.:129101-54-8
- Evodosin A
Catalog No.:BCN7322
CAS No.:1291053-38-7
- ENMD-2076 L-(+)-Tartaric acid
Catalog No.:BCC2185
CAS No.:1291074-87-7
- CGRP 8-37 (rat)
Catalog No.:BCC5717
CAS No.:129121-73-9
- 2-(2,2-Dimethyl-1,3-dioxolan-4-yl)propane-1,2-diol
Catalog No.:BCC8475
CAS No.:129141-48-6
Influence of cyclosporine and everolimus on the main mycophenolate mofetil pharmacokinetic parameters: Cross-sectional study.[Pubmed:28353583]
Medicine (Baltimore). 2017 Mar;96(13):e6469.
The objective of the present study was to assess the effect of cyclosporine (CsA) on the pharmacokinetic parameters of mycophenolic acid (MPA), an active Mycophenolate Mofetil (MMF) metabolite, and to compare with the effect of everolimus (EVR).Anonymized medical records of 404 kidney recipients were reviewed. The main MPA pharmacokinetic parameters (AUC(0-12) and Cmax) were evaluated.The patients treated with a higher mean dose of CsA displayed higher MPA AUC(0-12) exposure in the low-dose MMF group (1000 mg/day) (40.50 +/- 10.97 vs 28.08 +/- 11.03 h mg/L; rs = 0.497, P < 0.05), medium-dose MMF group (2000 mg/day) (43.00 +/- 6.27 vs 28.85 +/- 11.08 h mg/L; rs = 0.437, P < 0.01), and high-dose MMF group (3000 mg/day) (56.75 +/- 16.78 vs 36.20 +/- 3.70 h mg/L; rs = 0.608, P < 0.05).A positive correlation was also observed between the mean CsA dose and the MPA Cmax in the low-dose MMF group (Cmax 22.83 +/- 10.82 vs 12.08 +/- 5.59 mg/L; rs = 0.507, P < 0.05) and in the medium-dose MMF group (22.77 +/- 8.86 vs 13.00 +/- 6.82 mg/L; rs = 0.414, P < 0.01).The comparative analysis between 2 treatment arms (MMF + CsA and MMF + EVR) showed that MPA AUC(0-12) exposure was by 43% higher in the patients treated with a medium dose of MMF and EVR than in the patients treated with a medium dose of MMF and CsA.The data of the present study suggest a possible CsA versus EVR influence on MMF pharmacokinetics. Study results show that CsA has an impact on the main MPA pharmacokinetic parameters (AUC(0-12) and Cmax) in a CsA dose-related manner, while EVR mildly influence or does not affect MPA pharmacokinetic parameters. Low-dose CsA (lower than 180 mg/day) reduces MPA AUC(0-12) exposure under the therapeutic window and may lead to ineffective therapy, while a high-dose CsA (>240 mg/day) is related to greater than 10 mg/L MPA Cmax and increases the likelihood of adverse events.
Mycophenolate Mofetil and Rapamycin Induce Apoptosis in the Human Monocytic U937 Cell Line Through Two Different Pathways.[Pubmed:28345768]
J Cell Biochem. 2017 Oct;118(10):3480-3487.
Transplant vasculopathy may be considered as an accelerated form of atherosclerosis resulting in chronic rejection of vascularized allografts. After organ transplantation, a diffuse intimal thickening is observed, leading to the development of an atherosclerosis plaque due to a significant monocyte infiltration. This results from a chronic inflammatory process induced by the immune response. In this study, we investigated the impact of two immunosuppressive drugs used in therapy initiated after organ transplantation, Mycophenolate Mofetil, and rapamycin, on the apoptotic response of monocytes induced or not by oxidized LDL. Here we show the pro-apoptotic effect of these two drugs through two distinct signaling pathways and we highlight a synergistic effect of rapamycin on apoptosis induced by oxidized LDL. In conclusion, since immunosuppressive therapy using Mycophenolate Mofetil or rapamycin can increase the cell death in a monocyte cell line, this treatment could exert similar effects on human monocytes in transplant patients, and thus, prevent transplant vasculopathy, atherosclerosis development, and chronic allograft rejection. J. Cell. Biochem. 118: 3480-3487, 2017. (c) 2017 Wiley Periodicals, Inc.
The efficacy of mycophenolate mofetil in treating Takayasu arteritis: a systematic review and meta-analysis.[Pubmed:28364217]
Rheumatol Int. 2017 Jul;37(7):1083-1088.
The purpose of this study is to assess the effectiveness of Mycophenolate Mofetil (MMF) in treating Takayasu arteritis (TA) patients. Embase, Cochrane Library, Pubmed, Clinicaltrials. Gov and three Chinese literature databases (VIP, CNKI, WanFang) were searched; randomized-controlled trials and observational studies that compared the efficacy before and after treatment with MMF were included. The efficacy outcomes were disease activity, the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) values and steroid dosage. The results were expressed as mean differences with 95% confidence intervals. Compared with the baseline, there were significant reductions in the ESR (-14.92 [25.35, -4.48]), CRP values (-12.99 [-23.29, -2.68]) and the steroid dosage (-17.64 [-24.89, -10.4]) after the addition of MMF, and the disease tended to stabilize. Therefore, MMF might be an alternative immunosuppressive drug for TA for the control of disease activity and to taper the steroid dosage.
Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF).[Pubmed:8680053]
Clin Transplant. 1996 Feb;10(1 Pt 2):77-84.
Mycophenolate Mofetil (MMF) is a novel immunosuppressive drug that shows promise in preventing the rejection of organ allografts and in the treatment of ongoing rejection. Orally administered MMF is hydrolyzed by esterases in the intestine and blood to release mycophenolic acid (MPA), a potent, selective, noncompetitive inhibitor of the type 2 isoform of inosine monophosphate dehydroxygenase (IMPDH) expressed in activated human T and B lymphocytes. By inhibiting IMPDH, MPA depletes the pool of dGTP required for DNA synthesis. MPA has a more potent cytostatic effect on lymphocytes than on other cell types, and this is the principal mechanism by which immunosuppressive activity is exerted. MPA also depletes pools of GTP in human lymphocytes and monocytes, thereby inhibiting the synthesis of fucose- and mannose-containing saccharide components of membrane glycoproteins. These are recognized by the family of adhesion molecules termed selectins. By this mechanism, MPA could decrease the recruitment of lymphocytes and monocytes into sites of graft rejection. In addition to preventing allograft rejection, MMF suppresses graft-versus-host reactions in lethal and nonlethal murine models. MMF inhibits primary antibody responses more efficiently than secondary responses. MPA inhibits the proliferation of human B lymphocytes transformed by Epstein-Barr virus and is not mutagenic. Clinically attainable concentrations of MPA suppress the proliferation of human arterial smooth muscle cells. These two properties of MPA may decrease the risk of lymphoma development and proliferative arteriopathy in long-term recipients of MMF.


