OspemifeneCAS# 128607-22-7 |
- Zanamivir
Catalog No.:BCC4946
CAS No.:139110-80-8
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- Oseltamivir phosphate
Catalog No.:BCC4690
CAS No.:204255-11-8
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- Nucleozin
Catalog No.:BCC1811
CAS No.:341001-38-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128607-22-7 | SDF | Download SDF |
| PubChem ID | 3036505 | Appearance | Powder |
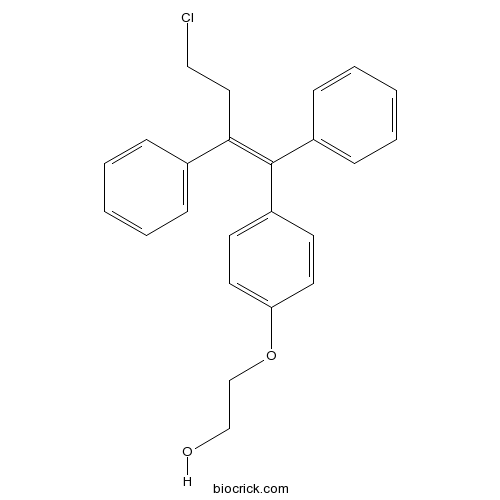
| Formula | C24H23ClO2 | M.Wt | 378.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (263.93 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]ethanol | ||
| SMILES | C1=CC=C(C=C1)C(=C(C2=CC=CC=C2)C3=CC=C(C=C3)OCCO)CCCl | ||
| Standard InChIKey | LUMKNAVTFCDUIE-VHXPQNKSSA-N | ||
| Standard InChI | InChI=1S/C24H23ClO2/c25-16-15-23(19-7-3-1-4-8-19)24(20-9-5-2-6-10-20)21-11-13-22(14-12-21)27-18-17-26/h1-14,26H,15-18H2/b24-23- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ospemifene Dilution Calculator

Ospemifene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6393 mL | 13.1964 mL | 26.3929 mL | 52.7858 mL | 65.9822 mL |
| 5 mM | 0.5279 mL | 2.6393 mL | 5.2786 mL | 10.5572 mL | 13.1964 mL |
| 10 mM | 0.2639 mL | 1.3196 mL | 2.6393 mL | 5.2786 mL | 6.5982 mL |
| 50 mM | 0.0528 mL | 0.2639 mL | 0.5279 mL | 1.0557 mL | 1.3196 mL |
| 100 mM | 0.0264 mL | 0.132 mL | 0.2639 mL | 0.5279 mL | 0.6598 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pingpeimine C
Catalog No.:BCN8411
CAS No.:128585-96-6
- ML167
Catalog No.:BCC5348
CAS No.:1285702-20-6
- GSK2578215A
Catalog No.:BCC6243
CAS No.:1285515-21-0
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
- Ophiogenin-3-O-alpha-L-rhaMnopyranosyl-(1→2)-beta-D-glucopyranoside
Catalog No.:BCN1587
CAS No.:128502-94-3
- Methylophioponanone B
Catalog No.:BCN6525
CAS No.:128446-36-6
- 2-Hydroxypropyl-β-cyclodextrin
Catalog No.:BCC6757
CAS No.:128446-35-5
- Gelidoside
Catalog No.:BCN7320
CAS No.:128420-44-0
- Euojaponine D
Catalog No.:BCC8980
CAS No.:128397-42-2
- Hydroprotopine
Catalog No.:BCN6155
CAS No.:128397-41-1
- Hyptadienic acid
Catalog No.:BCN6154
CAS No.:128397-09-1
- MCH (human, mouse, rat)
Catalog No.:BCC6068
CAS No.:128315-56-0
- Z(2-Br)-Osu
Catalog No.:BCC2806
CAS No.:128611-93-8
- PyBOP
Catalog No.:BCC2820
CAS No.:128625-52-5
- 1,6-O,O-Diacetylbritannilactone
Catalog No.:BCN7792
CAS No.:1286694-67-4
- FRAX597
Catalog No.:BCC4172
CAS No.:1286739-19-2
- Eucamalduside A
Catalog No.:BCN7321
CAS No.:1287220-29-4
- Kalopanaxsaponin H
Catalog No.:BCN2572
CAS No.:128730-82-5
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- Fmoc-D-Asp(OtBu)-OH
Catalog No.:BCC3471
CAS No.:12883-39-3
- Fargesol
Catalog No.:BCN6421
CAS No.:128855-64-1
- Maohuoside A
Catalog No.:BCN5348
CAS No.:128988-55-6
- Suramin hexasodium salt
Catalog No.:BCC7079
CAS No.:129-46-4
- Methysergide maleate
Catalog No.:BCC5698
CAS No.:129-49-7
Clinical update on the use of ospemifene in the treatment of severe symptomatic vulvar and vaginal atrophy.[Pubmed:27822125]
Int J Womens Health. 2016 Oct 26;8:617-626.
The physiological decrease in vaginal estrogens is accountable for the emergence of vulvar and vaginal atrophy (VVA) and its related symptoms such as vaginal dryness, dyspareunia, vaginal and/or vulvar irritation or itching, and dysuria. The repercussion of these symptoms on quality of life often makes it necessary to initiate treatment. Up until now, the treatments available included vaginal moisturizers and lubricants, local estrogens, and hormonal therapy. However, therapeutic options have now been increased with the approval of 60 mg Ospemifene, the first nonhormonal oral treatment with an agonist effect on the vaginal epithelium and an endometrial and breast safety profile which makes it unique. This is the first selective estrogen receptor modulator indicated in women with moderate-to-severe vaginal atrophy not eligible for local estrogen treatment. Considering that "local estrogen noneligible women" are those in whom such treatment cannot be administered either because it is contraindicated or due to skill issues, who are averse to the mode and convenience of vaginal products' administration or to their use on account of potential systemic absorption, or those who demonstrate dissatisfaction in terms of efficacy and safety, it is clear that there is a significant unmet medical need in VVA management. In fact, a great number of women show lack of adherence, dropping out of at least one VVA treatment, including nonhormonal moisturizers and lubricants, which they consider to be ineffective and uncomfortable. If they could choose, many of them may opt for oral treatment. In Phase III studies, Ospemifene demonstrated efficacy in vaginal dryness and dyspareunia, regenerating vaginal cells, improving lubrication, and reducing pain during sexual intercourse. Symptoms improved in the first 4 weeks and endured for up to 1 year. Additionally, it demonstrated a good endometrial, cardiovascular system, and breast safety profile.
Population pharmacokinetics of ospemifene and safety evaluation of pharmacokinetic alterations caused by intrinsic and extrinsic factors.[Pubmed:28128722]
Int J Clin Pharmacol Ther. 2017 Apr;55(4):339-347.
PURPOSE: To develop a population pharmacokinetic (PPK) model to assess factors influencing Ospemifene pharmacokinetics and to assess safety for pharmacokinetic alteration observed in drug development. METHOD: A PPK model was constructed using pooled Ospemifene concentrations. Covariates considered before start of the analysis were: age, race, body weight, BMI, albumin, alanine amino-transferase, bilirubin, and creatinine clearance. The expected distribution of Ospemifene concentration was derived for the 4 cases in phase-1 studies that increased Ospemifene exposure: administration to severe renal impairment subjects (case 1), administration to moderate hepatic impairment subjects (case 2), coadministration with ketoconazole (case 3), or coadministration with fluconazole (case 4). Safety information in a long-term safety trial was used to assess the potential changes in risk of adverse events with Ospemifene-exposure increase. RESULTS: The PPK parameter estimates were 9.16 L/h for CL/F, 34.3 L for V2/F, 16.4 L/h for Q/F, 250 L for V3/F, and 0.522 h-1 for ka, based on the final model. Distributions of estimated AUC in a phase-3 study largely covered the expected distribution for case 1, case 2, or case 3, but did not overlap the expected distribution for case 4. The incidences of adverse events were not associated with Ospemifene exposure in the long-term safety study. CONCLUSIONS: We developed an Ospemifene PPK model and identified no relevant covariate in the PPK analysis. The drug appears safe to use in renal impairment, moderate hepatic impairment, and when coadministered with ketoconazole. Ospemifene should not be administered with fluconazole..
Systematic indirect comparison of ospemifene versus local estrogens for vulvar and vaginal atrophy.[Pubmed:28267367]
Climacteric. 2017 Jun;20(3):195-204.
In the absence of a direct head-to-head study, we performed an indirect historical comparison of Ospemifene 60 mg (Senshio((R))) vs. local vaginal estrogens in moderate or severe vulvar and vaginal atrophy (VVA). A literature search was carried out of clinical efficacy/safety trials of local vaginal estrogens in VVA approved in Europe. For efficacy comparison, studies had to be placebo-controlled and of 12 weeks' duration. For safety comparison, studies had to be >/=40 weeks' duration. Efficacy endpoints were the difference between active and placebo in change from baseline to week 12 for symptoms, vaginal pH, and maturation value (MV). Safety endpoints were endometrial safety, breast safety, thrombosis, and adverse events. The 12-week improvement over placebo in symptom score was not different for Ospemifene 60 mg and 17beta-estradiol 10 mug and for Ospemifene 60 mg and estriol gel. After 12 weeks, the percentages with vaginal pH <5.0 and <5.5 were better for Ospemifene 60 mg than 10 mug 17beta-estradiol. Week-12 pH changes were comparable with estriol pessaries or gel and Ospemifene 60 mg. The 12-week MV improvements over placebo were similar or better with Ospemifene 60 mg compared with 10 mug 17beta-estradiol and with estriol pessaries or gel. There was no increased vaginal bleeding, endometrial hyperplasia, or carcinoma (including breast cancer) relative to placebo and no signal for increased risk of venous thromboembolism with Ospemifene 60 mg or 10 mug 17beta-estradiol, but the confidence intervals for both products do not exclude an increased risk. This historical indirect comparison suggests that Ospemifene 60 mg has an efficacy, safety, and tolerability profile comparable to or better than local vaginal estrogens in the treatment of VVA.
Repurposing ospemifene for potentiating an antigen-specific immune response.[Pubmed:27922937]
Menopause. 2017 Apr;24(4):437-451.
OBJECTIVE: Ospemifene, an estrogen receptor agonist/antagonist approved for the treatment of dyspareunia and vaginal dryness in postmenopausal women, has potential new indications as an immune modulator. The overall objective of the present series of preclinical studies was to evaluate the immunomodulatory activity of Ospemifene in combination with a peptide cancer vaccine. METHODS: Immune regulating effects, mechanism of action and structure activity relationships of Ospemifene and related compounds were evaluated by examining expression of T-cell activating cytokines in vitro, and antigen-specific immune response and cytotoxic T-lymphocyte activity in vivo. The effects of Ospemifene (OSP) on the immune response to a peptide cancer vaccine (PV) were evaluated after chronic [control (n = 22); OSP 50 mg/kg (n = 16); PV (n = 6); OSP+PV (n = 11)], intermittent [control (n = 10); OSP 10 and 50 mg/kg (n = 11); PV (n = 11); combination treatment (n = 11 each dose)] and pretreatment [control; OSP 100 mg/kg; PV 100 mug; combination treatment (n = 8 all groups)] Ospemifene oral dosing schedules in a total of 317 mixed-sex tumor-bearing and nontumor-bearing mice. RESULTS: The results showed that Ospemifene induced expression of the key TH1 cytokines interferon gamma and interleukin-2 in vitro, which may be mediated by stimulating T-cells through phosphoinositide 3-kinase and calmodulin signaling pathways. In combination with an antigen-specific peptide cancer vaccine, Ospemifene increased antigen-specific immune response and increased cytotoxic T-lymphocyte activity in tumor-bearing and nontumor-bearing mice. The pretreatment, intermittent, and chronic dosing schedules of Ospemifene activate naive T-cells, modulate antigen-induced tolerance and reduce tumor-associated, pro-inflammatory cytokines, respectively. CONCLUSIONS: Taken together, Ospemifene's dose response and schedule-dependent immune modulating activity offers a method of tailoring and augmenting the efficacy of previously failed antigen-specific cancer vaccines for a wide range of malignancies.


