ZanamivirInfluenza A/B virus neuraminidases inhibitor CAS# 139110-80-8 |
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139110-80-8 | SDF | Download SDF |
| PubChem ID | 60855 | Appearance | Powder |
| Formula | C12H20N4O7 | M.Wt | 332.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 33.33 mg/mL (100.30 mM) *"≥" means soluble, but saturation unknown. | ||
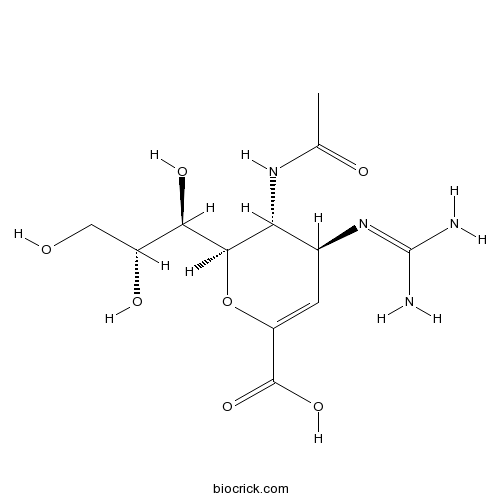
| Chemical Name | (2R,3R,4S)-3-acetamido-4-(diaminomethylideneamino)-2-[(1R,2R)-1,2,3-trihydroxypropyl]-3,4-dihydro-2H-pyran-6-carboxylic acid | ||
| SMILES | CC(=O)NC1C(C=C(OC1C(C(CO)O)O)C(=O)O)N=C(N)N | ||
| Standard InChIKey | ARAIBEBZBOPLMB-UFGQHTETSA-N | ||
| Standard InChI | InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Zanamivir is an influenza viral neuraminidase inhibitor with IC50 values of 0.95 nM and 2.7 nM for influenza A and B, respectively.In Vitro:Zanamivir interacts with a group of amino acids in the active site of neuraminidase, which are conserved in all influenza A and B strains. Zanamivir blocks the action of neuraminidase, which prevents the cleavage of sialic acid on the cell receptors, thus preventing release and spread of the newly formed virions[2].In Vivo:Zanamivir has a poor bioavailability in oral administration, with only 4–17% of the agent. Oral delivery of zanamivir has been a problem due to its strong hydrophilic nature that limits its transport across the intestinal epithelium. Permeation enhancers such as sodium cholate, hydroxypropyl β-cyclodextrin could be used with zanamivir to enhance the intestinal permeability[3]. References: | |||||

Zanamivir Dilution Calculator

Zanamivir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0092 mL | 15.0462 mL | 30.0924 mL | 60.1848 mL | 75.231 mL |
| 5 mM | 0.6018 mL | 3.0092 mL | 6.0185 mL | 12.037 mL | 15.0462 mL |
| 10 mM | 0.3009 mL | 1.5046 mL | 3.0092 mL | 6.0185 mL | 7.5231 mL |
| 50 mM | 0.0602 mL | 0.3009 mL | 0.6018 mL | 1.2037 mL | 1.5046 mL |
| 100 mM | 0.0301 mL | 0.1505 mL | 0.3009 mL | 0.6018 mL | 0.7523 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Zanamivir is a selective inhibitor of influenza A and B virus neuraminidases with IC50 values of 0.64-7.9nM [1].
Influenza A and B viruses are negative-strand RNA viruses. Neuraminidase is one of the two glycoproteins present on the surface of the virus. It is important to the pathogenicity and infectivity of the virus. The neuraminidases inhibitor, zanamivir, is a sialic acid analogue. It inhibits neuraminidases to cleave sialic acid on the surface of host cells and influenza viral envelope. In the in vitro assay, zanamivir suppresses the growth of influenza A and B viruses with IC50 values ranging from 5nM to 14nM for laboratory-passaged strains and from 20nM to 16μM for clinical isolates. In animal models infected with influenza A and B, treatment of zanamivir reduces the mortality and viral titres in lung homogenates and improves lung consolidation scores [1].
References:
[1] Elliott M. Zanamivir: from drug design to the clinic. Philos Trans R Soc Lond B Biol Sci, 2001, 356(1416): 1885-93.
- Vigabatrin Hydrochloride
Catalog No.:BCC5198
CAS No.:1391054-02-6
- PF 4800567 hydrochloride
Catalog No.:BCC7904
CAS No.:1391052-28-0
- Tristin
Catalog No.:BCN4709
CAS No.:139101-67-0
- (-)-Heraclenol
Catalog No.:BCN7682
CAS No.:139079-42-8
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- L-689,560
Catalog No.:BCC6774
CAS No.:139051-78-8
- Anemarsaponin B
Catalog No.:BCN6289
CAS No.:139051-27-7
- 2-PMDQ
Catalog No.:BCC6726
CAS No.:139047-55-5
- JMV 449
Catalog No.:BCC5863
CAS No.:139026-66-7
- Oplodiol
Catalog No.:BCN6204
CAS No.:13902-62-0
- Catalpin
Catalog No.:BCN6205
CAS No.:1390-72-3
- Carmine
Catalog No.:BCN2223
CAS No.:1390-65-4
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- Isomurralonginol acetate
Catalog No.:BCN4708
CAS No.:139115-59-6
- Tripterifordin
Catalog No.:BCN6206
CAS No.:139122-81-9
- Uralenol
Catalog No.:BCN7994
CAS No.:139163-15-8
- H-Tle-OH.HCl
Catalog No.:BCC2660
CAS No.:139163-43-2
- CEP-37440
Catalog No.:BCC5145
CAS No.:1391712-60-9
- ZM 241385
Catalog No.:BCC6902
CAS No.:139180-30-6
- Picfeltarraenin X
Catalog No.:BCN2859
CAS No.:1391826-61-1
- Verdinexor (KPT-335)
Catalog No.:BCC5573
CAS No.:1392136-43-4
- 24-Hydroxy-25-ethoxy-3,4-secocycloart-4(28)-en-3-oic acid methyl ester
Catalog No.:BCN7050
CAS No.:1392210-81-9
- Dodonaflavonol
Catalog No.:BCN6862
CAS No.:1392213-93-2
- 3,4-Dihydroxy-2-O-methylanigorufone
Catalog No.:BCN7182
CAS No.:1392307-42-4
Selection of multi-drug resistant influenza A and B viruses under zanamivir pressure and their replication fitness in ferrets.[Pubmed:28195559]
Antivir Ther. 2018;23(4):295-306.
BACKGROUND: Intravenous Zanamivir has been used to treat patients with severe influenza. Because the majority of cases (including immunocompromised patients) require the drug for an extended period of treatment, there is a higher risk that the virus will develop resistance. Therefore, knowing the possible amino acid substitutions that may arise in recently circulating influenza strains under prolonged Zanamivir exposure and their impact on antiviral susceptibility is important. METHODS: Influenza A(H1N1)pdm09, A(H3N2) and B virus were serially passaged under increasing Zanamivir pressure in vitro. Neuraminidase (NA) mutations that arose were introduced into recombinant viruses and the susceptibility to oseltamivir, Zanamivir, peramivir and laninamivir was determined. The replication fitness of the recombinant variants was assessed in the ferret. RESULTS: NA mutations E119D (N1 numbering) and E117D (B numbering) were detected in A(H1N1)pdm09 and B (Victoria-lineage) viruses respectively and were associated with reduced susceptibility to all four NA inhibitors. No NA mutations were detected in the A(H3N2) or B (Yamagata-lineage) viruses. In ferrets, the A(H1N1)pdm09 E119D variant caused a lower degree of morbidity and the mutation was found to be unstable with E119 reverted virus detected 4 days post-infection of ferrets with the variant E119D virus. In contrast, the influenza B E117D variant was genetically stable in ferrets, caused a noticeable level of morbidity but had a significant reduction in replication fitness compared to wild-type virus. CONCLUSIONS: The NA mutations E119D in influenza A(H1N1)pdm09 and E117D in influenza B viruses that arose under Zanamivir pressure conferred resistance to multiple NA inhibitors but had compromised viral replication in ferrets compared to wild-type virus without antiviral drug pressure.
Molecular modeling and lead design of substituted zanamivir derivatives as potent anti-influenza drugs.[Pubmed:28155702]
BMC Bioinformatics. 2016 Dec 22;17(Suppl 19):512.
BACKGROUND: Influenza virus spreads infection by two main surface glycoproteins, namely hemagglutinin (HA) and neuraminidase (NA). NA cleaves the sialic acid receptors eventually releasing newly formed virus particles which then invade new cells. Inhibition of NA could limit the replication of virus to one round which is insufficient to cause the disease. RESULTS: An experimentally reported series of acylguanidine Zanamivir derivatives was used to develop GQSAR model targeting NA in different strains of influenza virus, H1N1 and H3N2. A combinatorial library was developed and their inhibitory activities were predicted using the GQSAR model. CONCLUSION: The top leads were analyzed by docking which revealed the binding modes of these inhibitors in the active site of NA (150-loop). The top compound (AMA) was selected for carrying out molecular dynamics simulations for 15 ns which provided insights into the time dependent dynamics of the designed leads. AMA possessed a docking score of -8.26 Kcal/mol with H1N1 strain and -7.00 Kcal/mol with H3N2 strain. Ligand-bound complexes of both H1N1 and H3N2 were observed to be stable for 11 ns and 7 ns respectively. ADME descriptors were also calculated to study the pharmacokinetic properties of AMA which revealed its drug-like properties.
Intravenous zanamivir or oral oseltamivir for hospitalised patients with influenza: an international, randomised, double-blind, double-dummy, phase 3 trial.[Pubmed:28094141]
Lancet Respir Med. 2017 Feb;5(2):135-146.
BACKGROUND: Neuraminidase inhibitors are effective for the treatment of acute uncomplicated influenza. However, there is an unmet need for intravenous treatment for patients admitted to hospital with severe influenza. We studied whether intravenous Zanamivir was a suitable treatment in this setting. METHODS: In this international, randomised, double-blind, double-dummy, phase 3 trial, we recruited patients aged 16 years or older with severe influenza admitted to 97 hospitals from 26 countries. We randomly assigned patients (1:1:1 stratified by symptom onset Zanamivir, or standard-of-care (75 mg oral oseltamivir) twice a day for 5-10 days; patients were followed up for 28 days. The randomisation schedule, including stratification, was generated using GlaxoSmithKline's RandAll software. Patients, site study staff, and sponsor were masked to study treatment. The primary endpoint was time to clinical response-a composite of vital sign stabilisation and hospital discharge-in the influenza-positive population. The trial was powered to show an improvement of 1.5 days or greater with 600 mg intravenous Zanamivir. Pharmacokinetic, safety, and virology endpoints were also assessed. This trial is registered with ClinicalTrials.gov, number NCT01231620. FINDINGS: Between Jan 15, 2011, and Feb 12, 2015, 626 patients were randomly assigned to receive 300 mg intravenous Zanamivir (n=201), 600 mg intravenous Zanamivir (n=209), or 75 mg oral oseltamivir (n=205) twice a day; 11 patients discontinued the study before receiving any study treatment. 488 (78%) of 626 patients had laboratory-confirmed influenza. Compared with a median time to clinical response of 5.14 days in the 600 mg intravenous Zanamivir group, the median time to clinical response was 5.87 days (difference of -0.73 days, 95% CI -1.79 to 0.75; p=0.25) in the 300 mg intravenous Zanamivir group and 5.63 days (difference of -0.48 days, 95% CI -2.11 to 0.97; p=0.39) in the oseltamivir group. Four patients with influenza A/H1N1pdm09 in the oseltamivir group developed H275Y resistance mutations. Adverse events were reported in 373 (61%) of treated patients and were similar across treatment groups; the most common adverse events (300 mg intravenous Zanamivir, 600 mg intravenous Zanamivir, oseltamivir) were diarrhoea (10 [5%], 15 [7%], 14 [7%]), respiratory failure (11 [5%], 14 [7%], 11 [5%]), and constipation (7 [3%], 13 [6%], 10 [5%]). 41 (7%) treated patients died during the study (15 [7%], 15 [7%], 11 [5%]); the most common causes of death were respiratory failure and septic shock. INTERPRETATION: Time to clinical response to intravenous Zanamivir dosed at 600 mg was not superior to oseltamivir or 300 mg intravenous Zanamivir. All treatments had a similar safety profile in hospitalised patients with severe influenza. FUNDING: GlaxoSmithKline.
Development of oseltamivir and zanamivir resistance in influenza A(H1N1)pdm09 virus, Denmark, 2014.[Pubmed:28128091]
Euro Surveill. 2017 Jan 19;22(3). pii: 30445.
Antiviral treatment of immunocompromised patients with prolonged influenza virus infection can lead to multidrug resistance. This study reveals the selection of antiviral resistance mutations in influenza A(H1N1)pdm09 virus in an immunocompromised patient during a 6-month period. The patient was treated with two courses of oseltamivir (5 days and 2 months, respectively), with the first course starting at symptom onset, and subsequently Zanamivir (2 months and 10 days, respectively). Respiratory samples were investigated by Sanger and next generation sequencing (NGS) and, for NGS data, low-frequency-variant-detection analysis was performed. Neuraminidase-inhibition tests were conducted for samples isolated in Madin-Darby canine kidney cells. In a sample collected 15 days after the end of the first treatment with oseltamivir (Day 20 post-symptom onset), oseltamivir resistance was detected (mutation H275Y with 60.3% frequency by NGS). Day 149 when the patient had almost completed the second Zanamivir treatment, mixes of the following resistance mutations were detected; H275Y(65.1%), I223R(9.2%), and E119G(89.6%), accompanied by additional mutations, showing a more complex viral population in the long-term treated patient. Two samples obtained on Day 151 from bronchoalveolar lavage (BAL) and nasopharyngeal swab, respectively, showed different mutation profiles, with a higher frequency of antiviral resistance mutations in BAL. The results emphasise the importance of timely antiviral resistance testing both for treatment of individual patients as well as for preventive measures to control the development and transmission of antiviral resistant viruses.


