L-689,560potent NMDA antagonist CAS# 139051-78-8 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139051-78-8 | SDF | Download SDF |
| PubChem ID | 121918 | Appearance | Powder |
| Formula | C17H15Cl2N3O3 | M.Wt | 380.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in DMSO and to 100 mM in ethanol | ||
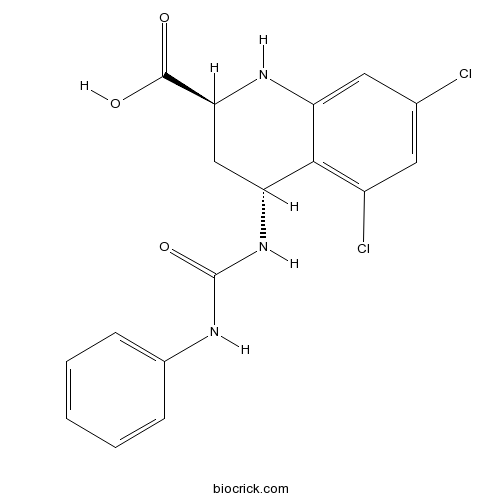
| Chemical Name | (2S,4R)-5,7-dichloro-4-(phenylcarbamoylamino)-1,2,3,4-tetrahydroquinoline-2-carboxylic acid | ||
| SMILES | C1C(C2=C(C=C(C=C2NC1C(=O)O)Cl)Cl)NC(=O)NC3=CC=CC=C3 | ||
| Standard InChIKey | UCKHICKHGAOGAP-KGLIPLIRSA-N | ||
| Standard InChI | InChI=1S/C17H15Cl2N3O3/c18-9-6-11(19)15-12(7-9)21-14(16(23)24)8-13(15)22-17(25)20-10-4-2-1-3-5-10/h1-7,13-14,21H,8H2,(H,23,24)(H2,20,22,25)/t13-,14+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Very potent antagonist at the glycine-NMDA site. Also available as part of the NMDA Receptor - Glycine Site. |

L-689,560 Dilution Calculator

L-689,560 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.63 mL | 13.1499 mL | 26.2999 mL | 52.5997 mL | 65.7497 mL |
| 5 mM | 0.526 mL | 2.63 mL | 5.26 mL | 10.5199 mL | 13.1499 mL |
| 10 mM | 0.263 mL | 1.315 mL | 2.63 mL | 5.26 mL | 6.575 mL |
| 50 mM | 0.0526 mL | 0.263 mL | 0.526 mL | 1.052 mL | 1.315 mL |
| 100 mM | 0.0263 mL | 0.1315 mL | 0.263 mL | 0.526 mL | 0.6575 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Kb: 130 nM
L-689,560 is a very potent antagonist at the glycine-NMDA site. The N-methyl-D-aspartate (NMDA) subtype of excitatorynamino acid receptor has been proved adequately that its relevant antagonists can reduce ischaemic brain damage (particularly in experimental models of focal cerebral ischaemia).
In vitro: L-689560 is described as one of the most potent NMDA antagonists and [4'-3H]-L-689560 has been thought to be a highly specific radioligand for the glycine site. In consistent with the 5,7-disubstituted kynurenates, the tetrahydroquinolines are selective antagonists of glycine site NMDA, L-689560 exhibiting at least 3 orders of magnitude selectivity versus the glutamate site [1].
In vivo: MDL100748 with an ED50 of 83 mg kg-1 can prevent audiogenic seizures in susceptible mice after systemic injection. As a standard L689560, its subsequent analogues have been compared; the displacement of [3H] L689560 has often been used to displace that of [3H] glycine as an alternative assay. L701252, a quinones (the retention of a keto grouping at position 3), has been against L689560 binding (IC50 of 420 nM) and against seizures (ED50 of 4.1 mg kg-1) in DBA/2 mice. A group of sulfonamide analogues of kynurenic acid are also in active among the 2-quinolone series. Those of a series of 3,4-dihydroquinolones and tetrahydroquinolines with a nitrosubstituent at 3-position were selective antagonists at the NMDA receptor glycine site if they bore a bulky grouping in the position 4. The compound with no substitution at position 4 was proved to be one of the most effective broad-spectrum antagonists against NMDA and AMPA receptors [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1]. Leeson PD, Carling RW, Moore KW, Moseley AM, Smith JD, Stevenson G, Chan T, Baker R, Foster AC, Grimwood S, et al. 4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor. J Med Chem. 1992 May 29;35 (11): 1954-68.
[2]. Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci. 2000 Apr; 21(4):149-54.
- Anemarsaponin B
Catalog No.:BCN6289
CAS No.:139051-27-7
- 2-PMDQ
Catalog No.:BCC6726
CAS No.:139047-55-5
- JMV 449
Catalog No.:BCC5863
CAS No.:139026-66-7
- Oplodiol
Catalog No.:BCN6204
CAS No.:13902-62-0
- Catalpin
Catalog No.:BCN6205
CAS No.:1390-72-3
- Carmine
Catalog No.:BCN2223
CAS No.:1390-65-4
- 3,4-Dihydroxybenzaldehyde
Catalog No.:BCN6214
CAS No.:139-85-5
- Ziprasidone hydrochloride monohydrate
Catalog No.:BCC2072
CAS No.:138982-67-9
- Capsazepine
Catalog No.:BCC1451
CAS No.:138977-28-3
- Coronarin D ethyl ether
Catalog No.:BCN6203
CAS No.:138965-89-6
- Isocoronarin D
Catalog No.:BCN6202
CAS No.:138965-88-5
- Rutaretin
Catalog No.:BCN4710
CAS No.:13895-92-6
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- (-)-Heraclenol
Catalog No.:BCN7682
CAS No.:139079-42-8
- Tristin
Catalog No.:BCN4709
CAS No.:139101-67-0
- PF 4800567 hydrochloride
Catalog No.:BCC7904
CAS No.:1391052-28-0
- Vigabatrin Hydrochloride
Catalog No.:BCC5198
CAS No.:1391054-02-6
- Zanamivir
Catalog No.:BCC4946
CAS No.:139110-80-8
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- Isomurralonginol acetate
Catalog No.:BCN4708
CAS No.:139115-59-6
- Tripterifordin
Catalog No.:BCN6206
CAS No.:139122-81-9
- Uralenol
Catalog No.:BCN7994
CAS No.:139163-15-8
- H-Tle-OH.HCl
Catalog No.:BCC2660
CAS No.:139163-43-2
- CEP-37440
Catalog No.:BCC5145
CAS No.:1391712-60-9
Catalytic enantioselective Reissert-type reaction: development and application to the synthesis of a potent NMDA receptor antagonist (-)-L-689,560 using a solid-supported catalyst.[Pubmed:11448184]
J Am Chem Soc. 2001 Jul 18;123(28):6801-8.
Full details of the first catalytic enantioselective Reissert-type reaction are described. Utilizing the Lewis acid-Lewis base bifunctional catalyst 5 or 6 (9 mol %), the Reissert products were obtained in 57 to 99% yield with 54 to 96% ee. Electron-rich quinolines produced better yields and enantioselectivities than electron-deficient substrates. Kinetic studies indicated that the reaction should proceed via the rate-determining acyl quinolinium formation, followed by the attack of a cyanide. The catalyst does not facilitate the first rate-determining step; however, it strongly facilitates the second cyanation step. The reaction was successfully applied to an efficient catalytic asymmetric synthesis of a potent NMDA receptor antagonist (-)-L-689,560. A key step is the one-pot process using the Reissert-type reaction from quinoline 1f, followed by stereoselective reduction of the resulting enamine 2f. This step gave the key intermediate 20 in 91% yield with 93% ee, using 1 mol % of 6. The enantiomerically pure target compound was obtained through 10 operations (including recrystallization) in total yield of 47%. Furthermore, 6 was immobilized to JandaJEL, and the resulting solid-supported catalyst 11 afforded 20 in a comparable yield to the homogeneous 6, but with slightly lower enantioselectivity.
Deficit of [3H]L-689,560 binding to the glycine site of the glutamate/NMDA receptor in the brain in Huntington's disease.[Pubmed:7964888]
J Neurol Sci. 1994 Aug;125(1):46-9.
Binding of [3H]L-689,560 to the glycine site of the glutamate/NMDA receptor was carried out using post-mortem brain tissue from patients with Huntington's disease (HD) and from matched controls. Decreased binding site density (by 62% in the caudate nucleus, 20% in the frontal cortex) was identified in HD. The deficit found in the caudate nucleus in HD correlated with deficits of GABA and glutamate concentrations and thus may reflect the ongoing disease process.
Polyamines modulate [3H]L-689,560 binding to the glycine site of the NMDA receptor from rat brain.[Pubmed:8137882]
Eur J Pharmacol. 1994 Jan 1;266(1):43-50.
The N-methyl-D-aspartate (NMDA) receptor complex possesses distinct recognition sites for glutamate, glycine and polyamines, which appear to be allosterically linked. We have investigated the effects of polyamines on the binding of the glycine site antagonist [3H](+/-)-4-(trans)-2-carboxy-5,7-dichloro-4-phenylaminocarbonylamino - 1,2,3,4-tetrahydroquinoline ([3H]L-689,560), using rat cortex/hippocampus P2 membranes. Spermine and spermidine partially inhibited [3H]L-689,560 binding under non-equilibrium conditions, with IC50 values of 25.9 and 106 microM, respectively. The putative polyamine site antagonists arcaine, 1,10-diaminodecane, diethylenetriamine and putrescine had no effect on [3H]L-689,560 binding per se at 1 mM. The inhibition of [3H]L-689,560 binding by spermine was antagonised by arcaine in a competitive manner, but not by 1,10-diaminodecane, diethylenetriamine or putrescine. Kinetic analysis revealed that spermine (100 microM) decreased the association and dissociation rates of [3H]L-689,560 binding. In saturation experiments 100 microM spermine increased the KD for [3H]L-689,560 binding from 1.99 nM to 4.03 nM, with no effect on the number of binding sites. Spermine increased the affinity of glycine site agonists in displacing [3H]L-689,560 binding, with no effect on inhibition by partial agonists or antagonists, suggesting that spermine promotes an 'agonist-preferring' state. Modulation of [3H]L-689,560 binding by agonists for the polyamine and glutamate sites on the NMDA receptor did not appear to be additive in nature.
Medium supplementation with zinc enables detection of imipramine-induced adaptation in glycine/NMDA receptors labeled with [3H]L-689,560.[Pubmed:17085868]
Pharmacol Rep. 2006 Sep-Oct;58(5):753-7.
Antidepressant drugs after chronic administration induce adaptive changes in the NMDA receptor complex. Radioligand-receptorbinding studies using [3H]5,7-dichlorokynurenic acid demonstrated a "down-regulation" of the glycine site/NMDA receptor following chronic treatment with antidepressants and electroconvulsive shock. However, binding procedure using this radioligand is time consuming because it requires the use of centrifugation method in the separation process. The introduction of a new radioligand of glycine/NMDA receptor, [3H]L-689,560 enables the application of a rapid filtration method. In the present study we demonstrate that 2-week treatment with imipramine (15 mg/kg ip) did not evoke alterations in specific [3H]L-689,560 binding and in IC50 value of glycine in displacing [3H]L-689,560 binding in the mouse or rat cortex. However, longer, a 4-week treatment with imipramine induced a significant 71% increase in IC50 value in displacing [3H]L-689.560 binding in the mouse cortex. Moreover, the presence of zinc in the incubation media, dose-dependently enhances detection of imipramine-induced increase in IC50 value of glycine in displacing [3H]L-689,560 binding in the rat cortex. The present data indicate that: (1) [3H]L-689,560 may be a suitable ligand for assessing adaptation of the glycine/NMDA sites and (2) the presence of zinc enhances detection of imipramine-induced reduction of glycine affinity for glycine/NMDA receptors labeled with [3H]L-689,560 which further indicates a significance of zinc in the mechanism of antidepressant treatment.
Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection.[Pubmed:10740291]
Trends Pharmacol Sci. 2000 Apr;21(4):149-54.
Manipulation of the kynurenine pathway of tryptophan metabolism has yielded a plethora of agents that are now being developed as neuroprotectants and anticonvulsants. This pathway is involved in the production of the excitotoxin quinolinic acid and the neuroprotectant kynurenic acid. Approaches used in the development of therapeutic agents include production of analogues or pro-drugs of kynurenic acid and inhibitors of the enzyme responsible for the synthesis of quinolinic acid. Indeed, analogues of the amino acid receptor antagonist kynurenic acid are now in, or are about to enter, clinical trials for stroke and related disorders. This review summarizes the mechanism of action of these various agents, the development of glutamate receptor antagonists from kynurenic acid and the range of their potential uses in neurology and psychiatry.
4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor.[Pubmed:1534584]
J Med Chem. 1992 May 29;35(11):1954-68.
trans-2-Carboxy-5,7-dichloro-4-amidotetrahydroquinolines, evolved from the lead 5,7-dichlorokynurenic acid, have been synthesized and tested for in vitro antagonist activity at the glycine site on the N-methyl-D-aspartate (NMDA) receptor. Optimization of the 4-substituent has provided antagonists having nanomolar affinity, including the urea trans-2-carboxy-5,7-dichloro-4[[(phenylamino)carbonyl]amino]-1,2,3, 4-tetrahydroquinoline (35; IC50 = 7.4 nM vs [3H]glycine binding; Kb = 130 nM for block of NMDA responses in the rat cortical slice), which is one of the most potent NMDA antagonists yet found. The absolute stereochemical requirements for binding were found to be 2S,4R, showing that, in common with other glycine-site NMDA receptor ligands, the unnatural configuration at the alpha-amino acid center is required. The preferred conformation of the trans-2,4-disubstituted tetrahydroquinoline system, as shown by X-ray crystallography and 1H NMR studies, places the 2-carboxyl pseudoequatorial and the 4-substituent pseudoaxial. Modifications of the 4-amide show that bulky substituents are tolerated and reveal the critical importance for activity of correct positioning of the carbonyl group. The high affinity of trans-2-carboxy-5,7-dichloro-4-[1-(3-phenyl-2-oxoimidazolidinyl)]- 1,2,3,4-tetrahydroquinoline (55; IC50 = 6 nM) suggests that the Z,Z conformer of the phenyl urea moiety in 35 is recognized by the receptor. Molecular modeling studies show that the 4-carbonyl groups of the kynurenic acids, the tetrahydroquinolines, and related antagonists based on N-(chlorophenyl)glycine, can interact with a single putative H-bond donor on the receptor. The results allow the establishment of a three-dimensional pharmacophore of the glycine receptor antagonist site, incorporating a newly defined bulk tolerance/hydrophobic region.


