TristinCAS# 139101-67-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139101-67-0 | SDF | Download SDF |
| PubChem ID | 15736297 | Appearance | Powder |
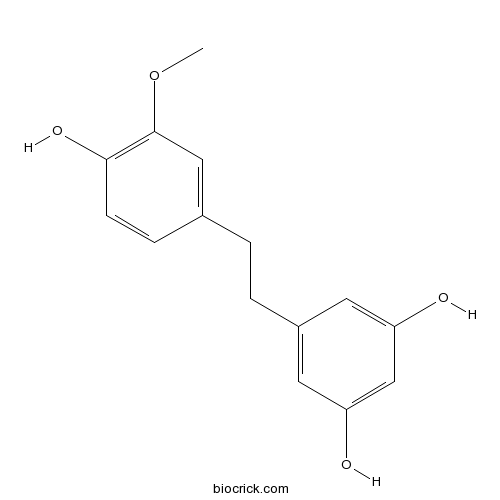
| Formula | C15H16O4 | M.Wt | 260.29 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[2-(4-hydroxy-3-methoxyphenyl)ethyl]benzene-1,3-diol | ||
| SMILES | COC1=C(C=CC(=C1)CCC2=CC(=CC(=C2)O)O)O | ||
| Standard InChIKey | KPFFMALTIRFAHW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H16O4/c1-19-15-8-10(4-5-14(15)18)2-3-11-6-12(16)9-13(17)7-11/h4-9,16-18H,2-3H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tristin shows stronger antioxidative activity than butylated hydroxyanisole (BHA). |

Tristin Dilution Calculator

Tristin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8419 mL | 19.2093 mL | 38.4187 mL | 76.8374 mL | 96.0467 mL |
| 5 mM | 0.7684 mL | 3.8419 mL | 7.6837 mL | 15.3675 mL | 19.2093 mL |
| 10 mM | 0.3842 mL | 1.9209 mL | 3.8419 mL | 7.6837 mL | 9.6047 mL |
| 50 mM | 0.0768 mL | 0.3842 mL | 0.7684 mL | 1.5367 mL | 1.9209 mL |
| 100 mM | 0.0384 mL | 0.1921 mL | 0.3842 mL | 0.7684 mL | 0.9605 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Heraclenol
Catalog No.:BCN7682
CAS No.:139079-42-8
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- L-689,560
Catalog No.:BCC6774
CAS No.:139051-78-8
- Anemarsaponin B
Catalog No.:BCN6289
CAS No.:139051-27-7
- 2-PMDQ
Catalog No.:BCC6726
CAS No.:139047-55-5
- JMV 449
Catalog No.:BCC5863
CAS No.:139026-66-7
- Oplodiol
Catalog No.:BCN6204
CAS No.:13902-62-0
- Catalpin
Catalog No.:BCN6205
CAS No.:1390-72-3
- Carmine
Catalog No.:BCN2223
CAS No.:1390-65-4
- 3,4-Dihydroxybenzaldehyde
Catalog No.:BCN6214
CAS No.:139-85-5
- Ziprasidone hydrochloride monohydrate
Catalog No.:BCC2072
CAS No.:138982-67-9
- Capsazepine
Catalog No.:BCC1451
CAS No.:138977-28-3
- PF 4800567 hydrochloride
Catalog No.:BCC7904
CAS No.:1391052-28-0
- Vigabatrin Hydrochloride
Catalog No.:BCC5198
CAS No.:1391054-02-6
- Zanamivir
Catalog No.:BCC4946
CAS No.:139110-80-8
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- Isomurralonginol acetate
Catalog No.:BCN4708
CAS No.:139115-59-6
- Tripterifordin
Catalog No.:BCN6206
CAS No.:139122-81-9
- Uralenol
Catalog No.:BCN7994
CAS No.:139163-15-8
- H-Tle-OH.HCl
Catalog No.:BCC2660
CAS No.:139163-43-2
- CEP-37440
Catalog No.:BCC5145
CAS No.:1391712-60-9
- ZM 241385
Catalog No.:BCC6902
CAS No.:139180-30-6
- Picfeltarraenin X
Catalog No.:BCN2859
CAS No.:1391826-61-1
- Verdinexor (KPT-335)
Catalog No.:BCC5573
CAS No.:1392136-43-4
Dendrobium protoplast co-culture promotes phytochemical assemblage in vitro.[Pubmed:27837285]
Protoplasma. 2017 Jul;254(4):1517-1528.
The present study is intended to analyze the occurrence of potent, low produce, naturally occurring stilbenes in protoplasts of wild species and hybrids of Dendrobium. The wild species selected for the study was Dendrobium ovatum, endemic to Western Ghats of India. Protoplasts were isolated from leaves and tepal tissues of all the species and were cultured purely to generate homofusants and cross-cultured to raise heterofusants. Phytochemical composition of protoplast culture with atypical and pure microcolonies was performed using mass spectrometry. Enzyme cocktail of 4% pectinase together with 2% cellulase displayed the highest competence for protoplast isolations. Maximum protoplast density of 30.11 x 10(4)/ml was obtained from D. ovatum leaves in 2 h. Subcellular features such as the presence of partially formed cell wall, the position of the nucleus, chloroplast density, colony existence, and integrity of the plasma membrane were analyzed. Among the pure and cross-cultured protoplasts, the number of heterofusants and homofusants formed were enumerated. The spectral feature extraction of the mass spectrometry indicated the presence of five phenolic marker compounds, viz., Tristin, confusarin, gigantol, moscatilin, and resveratrol, some of them in pure and others in assorted protoplast cultures raised from Dendrobium leaves and tepals. The study demonstrated that protoplast fusion technique enabled phytochemical assemblage in vitro as stilbenes tend to get restricted either in a tissue or species specific manner. This is the first report showing the presence of resveratrol, moscatilin, Tristin, gigantol, and confusarin in wild and hybrid species from cultured Dendrobium protoplasts in vitro.
A new (propylphenyl)bibenzyl from Eria bambusifolia.[Pubmed:26795438]
Nat Prod Res. 2016 Aug;30(15):1740-5.
A new (propylphenyl)bibenzyl, bambusifolol (1), along with six known compounds, batatasin III (2), Tristin (3), 3-hydroxy-5-methoxy bibenzyl (4), gigantol (5), 3',5-dimethoxy-9,9'-diacetyl-4,7'-epoxy-3,8'-bilign-7-ene-4'-methol (6) and balanophonin (7) were isolated from the whole plants of Eria bambusifolia. Their structures were elucidated by the means of extensive spectroscopic analysis. 3-7 were isolated from the genus Eria for the first time and 2 obtained originally from E. bambusifolia. All the compounds isolated were evaluated for their cytotoxicity against human tumour HL-60, SMMC-7721, A-549, MCF-7 and SW-480 cell lines, but none showed significant activity.
[Bibenzyl from Dendrobium inhibits angiogenesis and its underlying mechanism].[Pubmed:23724644]
Yao Xue Xue Bao. 2013 Mar;48(3):337-42.
Bibenzyl is a type of active compounds abundant in Dendrobium. In the present study, we investigated the inhibitory effects of six bibenzyls isolated from Dendrobium species on vascular endothelial growth factor (VEGF)-induced tube formation in human umbilical vascular endothelial cells (HUVECs). All those bibenzyls inhibited VEGF-induced tube formation at 10 micromol x L(-1) except Tristin, and of which moscatilin was found to have the strongest activity at the same concentration. The lowest effective concentration of moscatilin was 1 micromol x L(-1). Further results showed that moscatilin inhibited VEGF-induced capillary-like tube formation on HUVECs in a concentration-dependent manner. Western blotting results showed that moscatilin also inhibited VEGF-induced phosphorylation of VEGFR2 (Flk-1/KDR) and extracellular signal-regulated kinase 1/2 (ERK1/2). Further results showed that moscatilin inhibited VEGF-induced activation of c-Raf and MEK1/2, which are both upstream signals of ERK1/2. Taken together, results presented here demonstrated that moscatilin inhibited angiogenesis via blocking the activation of VEGFR2 (Flk-1/KDR) and c-Raf-MEK1/2-ERK1/2 signals.
[Chemical constituents from tubers of Dioscorea bulbifera].[Pubmed:19873780]
Zhongguo Zhong Yao Za Zhi. 2009 Jul;34(13):1679-82.
OBJECTIVE: To study the chemical constituents in the tubers of Dioscorea bulbifera. METHOD: Compounds were isolated and purified with silica gel, ODS and Sephadex LH-20 column chromatography, their structures were determined by using spectroscopic methods including MS and NMR. RESULT: Fourteen compounds were isolated and identified as stigmasterol (1), mono-arachidin (2), 1,7-bis-(4-hydroxyphenyl)-1E,4E,6E-heptatrien-3-one (3), behenic acid (4), demethyl batatasin IV (5), 2,3'-di-hydroxy-4',5'-dimethoxybibenzyl (6), diosbulbin B (7), diosbulbin D (8), docosyl ferulate (9), 7-bis-(4-hydroxyphenyl) -4E, 6E-heptadien-3-one (10), 5,3,4-trihydroxy-3,7-dimethoxyflavone (11), Tristin(12), protocatechuic acid (13), adenosine (14). CONCLUSION: Compounds 24, 6, 9, 10, 12, 14 were isolated from the genus Dioscorea for the first time.
[Phenolic components from herbs of Dendrobium aphyllum].[Pubmed:19294851]
Zhongguo Zhong Yao Za Zhi. 2008 Dec;33(24):2922-5.
OBJECTIVE: To study the phenolic constituents of Dendrobium aphyllum. METHOD: Various chromatographic techniques were used to isolate and purify the constituents, their physico-chemical properties and spectral data were employed to elucidate their structures. RESULT: Nine bibenzyls and two benzylethanyl compounds were isolated and identified as: moscatilin (1), gigantol (2), batatasin (3), Tristin (4), 3, 5, 4'-trihydroxylbibenzyl (5), 3, 5-dimethoxyl-4, 4'-dihydroxylbibenzyl (6), moscatin (7), 2, 4, 7-trihydroxyl-9, 10-dihydrophenanthrene (8), hircinol (9), 2-(4-hydroxyphenyl) ethyl-beta-D-glucopyranoside, salidroside (10) and p-hydroxylbenzylacetic acid (11). CONCLUSION: All compounds were obtained firstly from the plant, and the compounds 10 and 11 were isolated in this genus for the first time.
Two novel bibenzyls from Dendrobium trigonopus.[Pubmed:18636377]
J Asian Nat Prod Res. 2008 Jul-Aug;10(7-8):653-7.
Two novel bibenzyl trigonopols A (1) and B (2), together with seven known compounds, gigantol (3), Tristin (4), moscatin (5), hircinol (6), naringenin (7), 3-(4-hydroxy-3-methoxyphenyl)-2-propen-1-ol (8), and ( - )-syringaresinol (9), have been isolated from the stems of Dendrobium trigonopus, of which compounds 6, 8, and 9 were isolated for the first time from this species. The structures of two new compounds were elucidated as threo-22-(17-hydroxyl-9-(3-hydroxyl-4-methoxy-phenethyl)-13,16,18-trimethoxy-21H- benzo[c]chromen-21-yl)ethane-22, 23-diol (1) and 9-(4-hydroxyl-3-methoxy-phenethyl)-17-(21-hydroxyl-20-methoxy-phenyl)chroman-11, 16-diol (2) on the basis of spectroscopic methods. Trigonopol A was found to exhibit antiplatelet aggregation activity in vitro with 67.55% inhibitory ration at 1.4337 x 10(- 3) M.


