DAPT (GSI-IX)γ-secretase inhibitor,potent and specific CAS# 208255-80-5 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- LY-900009
Catalog No.:BCC2103
CAS No.:209984-68-9
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 208255-80-5 | SDF | Download SDF |
| PubChem ID | 5311272 | Appearance | Powder |
| Formula | C23H26F2N2O4 | M.Wt | 432.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | gamma-Secretase Inhibitor IX, DAPT, GSI-IX | ||
| Solubility | DMSO : ≥ 100 mg/mL (231.24 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
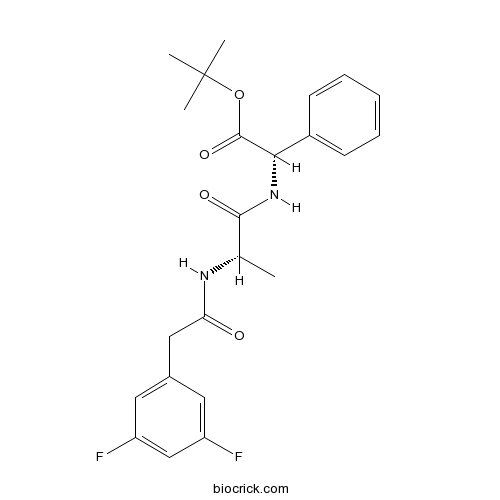
| Chemical Name | tert-butyl (2S)-2-[[(2S)-2-[[2-(3,5-difluorophenyl)acetyl]amino]propanoyl]amino]-2-phenylacetate | ||
| SMILES | CC(C(=O)NC(C1=CC=CC=C1)C(=O)OC(C)(C)C)NC(=O)CC2=CC(=CC(=C2)F)F | ||
| Standard InChIKey | DWJXYEABWRJFSP-XOBRGWDASA-N | ||
| Standard InChI | InChI=1S/C23H26F2N2O4/c1-14(26-19(28)12-15-10-17(24)13-18(25)11-15)21(29)27-20(16-8-6-5-7-9-16)22(30)31-23(2,3)4/h5-11,13-14,20H,12H2,1-4H3,(H,26,28)(H,27,29)/t14-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of γ-secretase; causes a reduction in Aβ40 and Aβ42 levels in human primary neuronal cultures (IC50 values are 115 and 200 nM for total Aβ and Aβ42 respectively) and in brain extract, cerebrospinal fluid and plasma in vivo. Does not affect APPα and APPβ levels. Blocks Notch signaling in hybrid human-mouse fetal thymus organ culture (FTOC). Activity causes ESCs to commit to neuronal differentiation. |

DAPT (GSI-IX) Dilution Calculator

DAPT (GSI-IX) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3124 mL | 11.5618 mL | 23.1235 mL | 46.2471 mL | 57.8088 mL |
| 5 mM | 0.4625 mL | 2.3124 mL | 4.6247 mL | 9.2494 mL | 11.5618 mL |
| 10 mM | 0.2312 mL | 1.1562 mL | 2.3124 mL | 4.6247 mL | 5.7809 mL |
| 50 mM | 0.0462 mL | 0.2312 mL | 0.4625 mL | 0.9249 mL | 1.1562 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2312 mL | 0.4625 mL | 0.5781 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DAPT, N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl Ester, is a potent and specific inhibitor of γ-secretase, a multimeric membrane protein complex that catalyzes proteolytic cleavage of amyloid precursor protein (APP) resulting in the accumulation of amyloi-β (Aβ) peptides which is associated with early on-set of familial Alzheimer’s disease (AD). It directly binds to the C-terminal fragment of the catalytic center of γ-secretase, presenilin (PS), especially within the transmenbrane domain 7 or more C-terminal region, resulting in the synthesis of a photoactivable DAPT derivative. Through oral administration, DAPT dose-dependently reduced Aβ peptides levels in vivo in Plasma and cerebrospinal fluid in young (6 months old, plaque-free) and aged (17 months old, plaque-bearing) Tg2576 mice.
Reference
Thomas A. Lanz, Carol S. Himes, Giovanni Pallante, Lisa Adams, Shinji Yamazaki, Ben Amore, and Kalpana M. Merchant. The γ-secretase inhibitor N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl Ester reduces Aβ levels in vivo in plasma and cerebrospinal fluid in young (plague-free) and aged (plaque-bearing) Tg2576 mice. The Journal of Pharmacology and Experimental Therapeutics 2003: 305 (3) 864-871
Yuichi Morohashi, Toshiyuki Kan, Yusuke Tominari, Haruhiko Fuwa, Yumiko Okamura, Naoto Watanabe, Chihiro Sato, Hideaki Natsugari, Tohru Fukuyama, Takeshi Iwatsubo, and Taisuke Tomita. C-terminal fragment of presenilin is the molecular target of a dipeptidic γ-secretase-specific inhibitor DAPT (N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl Ester). The Journal of Biological Chemistry 2006: 281(21) 14670-14676
- Nocistatin (bovine)
Catalog No.:BCC5703
CAS No.:208253-85-4
- Strictosidine
Catalog No.:BCN2641
CAS No.:20824-29-7
- Z-Ser(Bzl)-OH
Catalog No.:BCC2742
CAS No.:20806-43-3
- Hemokinin 1 (mouse)
Catalog No.:BCC5774
CAS No.:208041-90-1
- 4'-O-Methylbroussochalcone B
Catalog No.:BCN4908
CAS No.:20784-60-5
- Isobavachalcone
Catalog No.:BCN5415
CAS No.:20784-50-3
- Propofol
Catalog No.:BCC9130
CAS No.:2078-54-8
- 1,7-Bis(4-hydroxyphenyl)-3-hydroxy-1,3-heptadien-5-one
Catalog No.:BCN6470
CAS No.:207792-17-4
- 5-hydroxymethyl Tolterodine (PNU 200577, 5-HMT, 5-HM)
Catalog No.:BCC4583
CAS No.:207679-81-0
- HS 014
Catalog No.:BCC5819
CAS No.:207678-81-7
- TPT-260 Dihydrochloride
Catalog No.:BCC5172
CAS No.:2076-91-7
- H-Phe(4-NO2)-OH.H2O
Catalog No.:BCC3273
CAS No.:207591-86-4
- Zooxanthellabetaine A
Catalog No.:BCN1771
CAS No.:208256-89-7
- ZM336372
Catalog No.:BCC3875
CAS No.:208260-29-1
- Digoxin
Catalog No.:BCN5359
CAS No.:20830-75-5
- Daunorubicin
Catalog No.:BCC4115
CAS No.:20830-81-3
- Gentiopicroside
Catalog No.:BCN4909
CAS No.:20831-76-9
- Ethyl 4-(rhamnosyloxy)benzylcarbamate
Catalog No.:BCN7635
CAS No.:208346-80-9
- Stigmastane-3,5,6-triol
Catalog No.:BCN4910
CAS No.:20835-91-0
- Protoaescigenin
Catalog No.:BCC8240
CAS No.:20853-07-0
- Micafungin sodium
Catalog No.:BCC1750
CAS No.:208538-73-2
- H-Tle-OH
Catalog No.:BCC2659
CAS No.:20859-02-3
- Primulic Acid 2
Catalog No.:BCC8237
CAS No.:208599-88-6
- Berberine
Catalog No.:BCN4911
CAS No.:2086-83-1
NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell.[Pubmed:27108536]
Sci Rep. 2016 Apr 25;6:24704.
Cancer stem cells (CSCs) are considered responsible for tumor initiation and chemoresistance. This study was aimed to investigate the possibility of targeting head neck squamous cell carcinoma (HNSCC) by NOTCH1 pathway inhibition and explore the synergistic effect of combining NOTCH inhibition with conventional chemotherapy. NOTCH1/HES1 elevation was found in human HNSCC, especially in tissue post chemotherapy and lymph node metastasis, which is correlated with CSCs markers. NOTCH1 inhibitor DAPT (GSI-IX) significantly reduces CSCs population and tumor self-renewal ability in vitro and in vivo. Flow cytometry analysis showed that NOTCH1 inhibition reduces CSCs frequency either alone or in combination with chemotherapeutic agents, namely, cisplatin, docetaxel, and 5-fluorouracil. The combined strategy of NOTCH1 blockade and chemotherapy synergistically attenuated chemotherapy-enriched CSC population, promising a potential therapeutic exploitation in future clinical trial.
Blocking the NOTCH pathway can inhibit the growth of CD133-positive A549 cells and sensitize to chemotherapy.[Pubmed:24502949]
Biochem Biophys Res Commun. 2014 Feb 21;444(4):670-5.
Cancer stem cells (CSCs) are believed to play an important role in tumor growth and recurrence. These cells exhibit self-renewal and proliferation properties. CSCs also exhibit significant drug resistance compared with normal tumor cells. Finding new treatments that target CSCs could significantly enhance the effect of chemotherapy and improve patient survival. Notch signaling is known to regulate the development of the lungs by controlling the cell-fate determination of normal stem cells. In this study, we isolated CSCs from the human lung adenocarcinoma cell line A549. CD133 was used as a stem cell marker for fluorescence-activated cell sorting (FACS). We compared the expression of Notch signaling in both CD133+ and CD133- cells and blocked Notch signaling using the gamma-secretase inhibitor DAPT (GSI-IX). The effect of combining GSI and cisplatin (CDDP) was also examined in these two types of cells. We observed that both CD133+ and CD133- cells proliferated at similar rates, but the cells exhibited distinctive differences in cell cycle progression. Few CD133+ cells were observed in the G2/M phase, and there were half as many cells in S phase compared with the CD133- cells. Furthermore, CD133+ cells exhibited significant resistance to chemotherapy when treated with CDDP. The expression of Notch signaling pathway members, such as Notch1, Notch2 and Hes1, was lower in CD133+ cells. GSI slightly inhibited the proliferation of both cell types and exhibited little effect on the cell cycle. The inhibitory effects of DPP on these two types of cells were enhanced when combined with GSI. Interestingly, this effect was especially significant in CD133+ cells, suggesting that Notch pathway blockade may be a useful CSC-targeted therapy in lung cancer.
The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling.[Pubmed:17295317]
Dev Dyn. 2007 Mar;236(3):886-92.
During development of the neural tube, inhibition of the Notch response as well as the activation of the Sonic Hedgehog (Shh) response results in the formation of neuronal cell types. To determine whether Shh and Notch act independently, we tested the effects of the Notch inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) on neuralized, embryonic stem (ES) cell-derived embryoid bodies (EBs), while varying the levels of Shh pathway activation. Shh-resistant EBs were derived from Smo null ES cells, while EBs with constitutive high level of Shh pathway activation were derived from Ptc1 null ES cells. Intermediate levels of Shh pathway activation was achieved by the addition of ShhN to the EB culture medium. It was found that DAPT-mediated inhibition of the Notch response resulted in enhanced neuronal differentiation. In the absence of Shh, more interneurons were detected, while the main effect of DAPT on EBs with an activated Shh response was the precocious loss of ventral neuronal precursor-specific markers.
Different thresholds of Notch signaling bias human precursor cells toward B-, NK-, monocytic/dendritic-, or T-cell lineage in thymus microenvironment.[Pubmed:16030192]
Blood. 2005 Nov 15;106(10):3498-506.
Notch receptors are involved in lineage decisions in multiple developmental scenarios, including hematopoiesis. Here, we treated hybrid human-mouse fetal thymus organ culture with the gamma-secretase inhibitor 7 (N-[N-(3,5-difluorophenyl)-l-alanyl]-S-phenyl-glycine t-butyl ester) (DAPT) to establish the role of Notch signaling in human hematopoietic lineage decisions. The effect of inhibition of Notch signaling was studied starting from cord blood CD34(+) or thymic CD34(+)CD1(-), CD34(+)CD1(+), or CD4ISP progenitors. Treatment of cord blood CD34(+) cells with low DAPT concentrations results in aberrant CD4ISP and CD4/CD8 double-positive (DP) thymocytes, which are negative for intracellular T-cell receptor beta (TCRbeta). On culture with intermediate and high DAPT concentrations, thymic CD34(+)CD1(-) cells still generate aberrant intracellular TCRbeta(-) DP cells that have undergone DJ but not VDJ recombination. Inhibition of Notch signaling shifts differentiation into non-T cells in a thymic microenvironment, depending on the starting progenitor cells: thymic CD34(+)CD1(+) cells do not generate non-T cells, thymic CD34(+)CD1(-) cells generate NK cells and monocytic/dendritic cells, and cord blood CD34(+)Lin(-) cells generate B, NK, and monocytic/dendritic cells in the presence of DAPT. Our data indicate that Notch signaling is crucial to direct human progenitor cells into the T-cell lineage, whereas it has a negative impact on B, NK, and monocytic/dendritic cell generation in a dose-dependent fashion.
Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain.[Pubmed:11145990]
J Neurochem. 2001 Jan;76(1):173-81.
Converging lines of evidence implicate the beta-amyloid peptide (Ass) as causative in Alzheimer's disease. We describe a novel class of compounds that reduce A beta production by functionally inhibiting gamma-secretase, the activity responsible for the carboxy-terminal cleavage required for A beta production. These molecules are active in both 293 HEK cells and neuronal cultures, and exert their effect upon A beta production without affecting protein secretion, most notably in the secreted forms of the amyloid precursor protein (APP). Oral administration of one of these compounds, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester, to mice transgenic for human APP(V717F) reduces brain levels of Ass in a dose-dependent manner within 3 h. These studies represent the first demonstration of a reduction of brain A beta in vivo. Development of such novel functional gamma-secretase inhibitors will enable a clinical examination of the A beta hypothesis that Ass peptide drives the neuropathology observed in Alzheimer's disease.


