HS 014Selective MC4 receptor antagonist CAS# 207678-81-7 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 207678-81-7 | SDF | Download SDF |
| PubChem ID | 102600977 | Appearance | Powder |
| Formula | C71H94N20O17S2 | M.Wt | 1563.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
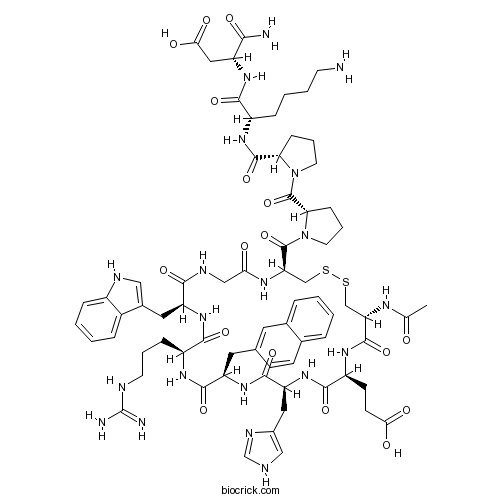
| Sequence | CEHXRWGCPPKD (Modifications: Cys-1 = N-terminal Ac, X = 2-Nal, Asp-12 = C-terminal amide, Disulfide bridge between 1 - 8) | ||
| SMILES | CC(=O)NC1CSSCC(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC1=O)CCC(=O)O)CC2=CNC=N2)CC3=CC4=CC=CC=C4C=C3)CCCNC(=N)N)CC5=CNC6=CC=CC=C65)C(=O)N7CCCC7C(=O)N8CCCC8C(=O)NC(CCCCN)C(=O)NC(CC(=O)O)C(=O)N | ||
| Standard InChIKey | BRGDPINZISNYJL-FFEBNQEQSA-N | ||
| Standard InChI | InChI=1S/C71H94N20O17S2/c1-38(92)81-53-35-109-110-36-54(69(107)91-26-10-18-56(91)70(108)90-25-9-17-55(90)68(106)85-46(15-6-7-23-72)62(100)86-49(60(73)98)31-59(96)97)82-57(93)34-79-61(99)51(29-42-32-78-45-14-5-4-13-44(42)45)88-63(101)47(16-8-24-77-71(74)75)83-65(103)50(28-39-19-20-40-11-2-3-12-41(40)27-39)87-66(104)52(30-43-33-76-37-80-43)89-64(102)48(84-67(53)105)21-22-58(94)95/h2-5,11-14,19-20,27,32-33,37,46-56,78H,6-10,15-18,21-26,28-31,34-36,72H2,1H3,(H2,73,98)(H,76,80)(H,79,99)(H,81,92)(H,82,93)(H,83,103)(H,84,105)(H,85,106)(H,86,100)(H,87,104)(H,88,101)(H,89,102)(H,94,95)(H,96,97)(H4,74,75,77)/t46-,47-,48-,49+,50+,51-,52-,53-,54+,55-,56-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective melanocortin MC4 receptor antagonist (Ki values are 3.16, 108, 54.4 and 694 nM for cloned human MC4, MC1, MC3 and MC5 receptors respectively). Increases food intake in rats and nociception in mice following central administration in vivo. Also inhibits IL-1β-induced Fos expression in the paraventricular hypothalamus. |

HS 014 Dilution Calculator

HS 014 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TPT-260 Dihydrochloride
Catalog No.:BCC5172
CAS No.:2076-91-7
- H-Phe(4-NO2)-OH.H2O
Catalog No.:BCC3273
CAS No.:207591-86-4
- SB 203186 hydrochloride
Catalog No.:BCC5673
CAS No.:207572-69-8
- iso-PPADS tetrasodium salt
Catalog No.:BCC6749
CAS No.:207572-67-6
- Pirlindole mesylate
Catalog No.:BCC6764
CAS No.:207572-66-5
- (±)-1-(1,2-Diphenylethyl)piperidine maleate
Catalog No.:BCC6619
CAS No.:207461-99-2
- BTCP maleate
Catalog No.:BCC6753
CAS No.:207455-25-2
- L-693,403 maleate
Catalog No.:BCC5657
CAS No.:207455-21-8
- N-MPPP Hydrochloride
Catalog No.:BCC5672
CAS No.:207452-97-9
- Arteannuin N
Catalog No.:BCN4907
CAS No.:207446-92-2
- Arteannuin M
Catalog No.:BCN4906
CAS No.:207446-90-0
- Arteannuin L
Catalog No.:BCN4905
CAS No.:207446-89-7
- 5-hydroxymethyl Tolterodine (PNU 200577, 5-HMT, 5-HM)
Catalog No.:BCC4583
CAS No.:207679-81-0
- 1,7-Bis(4-hydroxyphenyl)-3-hydroxy-1,3-heptadien-5-one
Catalog No.:BCN6470
CAS No.:207792-17-4
- Propofol
Catalog No.:BCC9130
CAS No.:2078-54-8
- Isobavachalcone
Catalog No.:BCN5415
CAS No.:20784-50-3
- 4'-O-Methylbroussochalcone B
Catalog No.:BCN4908
CAS No.:20784-60-5
- Hemokinin 1 (mouse)
Catalog No.:BCC5774
CAS No.:208041-90-1
- Z-Ser(Bzl)-OH
Catalog No.:BCC2742
CAS No.:20806-43-3
- Strictosidine
Catalog No.:BCN2641
CAS No.:20824-29-7
- Nocistatin (bovine)
Catalog No.:BCC5703
CAS No.:208253-85-4
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- Zooxanthellabetaine A
Catalog No.:BCN1771
CAS No.:208256-89-7
- ZM336372
Catalog No.:BCC3875
CAS No.:208260-29-1
Central blockade of melanocortin receptors attenuates the metabolic and locomotor responses to peripheral interleukin-1beta administration.[Pubmed:18082228]
Neuropharmacology. 2008 Mar;54(3):509-20.
Loss of appetite and cachexia is an obstacle in the treatment of chronic infection and cancer. Proinflammatory cytokines released from activated immune cells and acting in the central nervous system (CNS) are prime candidates for mediating these metabolic changes, potentially affecting both energy intake as well as energy expenditure. The effect of intravenous administration of two proinflammatory cytokines, interleukin (IL)-1beta (15 microg/kg) and tumor necrosis factor (TNF)-alpha (10 microg/kg), on food and water intake, locomotor activity, oxygen consumption (VO2), and respiratory exchange ratio (RER) was evaluated. The two cytokines elicited a comparable decrease in food intake and activated similar numbers of cells in the paraventricular nucleus of the hypothalamus (PVH), a region that plays a critical role in the regulation of appetite and metabolism (determined via expression of the immediate early gene, c-fos). However, only IL-1beta reduced locomotion and RER, and increased VO2, while TNF-alpha was without effect. To examine the role of the melanocortins in mediating IL-1beta- induced metabolic changes, animals were pretreated centrally with a melanocortin receptor antagonist, HS014. Pretreatment with HS014 blocked the effect of IL-1beta on food intake and RER at later time points (beyond 8 h post injection), as well as the hypoactivity and increased metabolic rate. Further, HS014 blocked the induction of Fos-ir in the PVH. These data highlight the importance of the melanocortin system, particularly within the PVH, in mediating a broad range of metabolic responses to IL-1beta.
Melanocortin receptor agonists and antagonists modulate nociceptive sensitivity in the mouse formalin test.[Pubmed:14660013]
Eur J Pharmacol. 2003 Dec 15;482(1-3):127-32.
A number of studies suggest the involvement of melanocortins in nociception, and although the mechanism through which this occurs is still unknown, experimental evidence would suggest an involvement of melanocortin MC(4) receptors. We investigated the effect of melanocortin receptor agonist and antagonists on nociceptive behaviour induced by formalin in the mouse. The intrathecal injection of the melanocortin receptor agonist MTII ([Ac-Nle(4),Asp(5),D-Phe(7),Lys(10)]cyclo-alpha-MSH-(4-10) amide) (5 nmol; P<0.05) significantly increased nociception in both phases of the formalin test, whereas the synthetic melanocortin receptor antagonists, SHU9119 ([Ac-Nle(4),Asp(5),D-2-Nal(7),Lys(10)]cyclo-alpha-MSH-(4-10) amide) (5 nmol), HS014 ([Ac-Cys(11),D-2-Nal(14),Cys(18)]beta-MSH-(11-22)amide) (5 nmol), and JKC-363 (cyclic [Mpr(11),D-Nal(14),Cys(18),Asp(22)-NH(2)]beta-MSH-11-22)) (5 nmol), and the endogenous receptor antagonist Agouti-related protein (AgRP) (1.5 nmol) were effective in reducing nociception in the late phase of the formalin test (50-60% of reduction in licking/flinching response; P<0.05). The present findings further support the involvement of the melanocortin system in the control of nociception. Moreover, considering that melanocortin MC(4) receptors are the only melanocortin subtype receptors present in the spinal cord, we can assume that the activity of the peptides in the formalin model is mediated through melanocortin MC(4) receptors.
Discovery of novel melanocortin4 receptor selective MSH analogues.[Pubmed:9630346]
Br J Pharmacol. 1998 May;124(1):75-82.
1. We synthesized a novel series of cyclic melanocyte stimulating hormone (MSH) analogues and tested their binding properties on cells transiently expressing the human melanocortin1 (MC1), MC3, MC4 and MC5 receptors. 2. We discovered that compounds with 26 membered rings of [Cys4,D-Nal7,Cys11]alpha-MSH(4-11) displayed specific MC4 receptor selectivity. The preference order of the different MC receptor subtypes for the novel [Cys4D-Nal7Cys11]alpha-MSH(4-11) analogues are distinct from all other known MSH analogues, particularly as they bind the MC4 receptor with high and the MC1 receptor with low relative affinities. 3. HS964 and HS014 have 12 and 17 fold MC4/MC3 receptor selectivity, respectively, which is much higher than for the previously described cyclic lactam and [Cys4,Cys10]alpha-MSH analogues SHU9119 and HS9510. 4. HS964 is the first substance showing higher affinity for the MC5 receptor than the MC1 receptor. 5. HS014, which was the most potent and selective MC4 receptor ligand (Ki 3.2 nM, which is approximately 300 fold higher affinity than for alpha-MSH), was also demonstrated to antagonize alpha-MSH stimulation of cyclic AMP in MC4 receptor transfected cells. 6. We found that a compound with a 29 membered ring of [Cys3,Nle10,D-Nal7,Cys11]alpha-MSH(3-11) (HS010) had the highest affinity for the MC3 receptor. 7. This is the first study to describe ligands that are truly MC4 selective and a ligand having a high affinity for the MC3 receptor. The novel compounds may be of use in clarifying the physiological roles of the MC3, MC4 and MC5 receptors.


