Begacestat╬│-secretase inhibitor CAS# 769169-27-9 |
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- PMSF
Catalog No.:BCC1229
CAS No.:329-98-6
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- Aprotinin
Catalog No.:BCC1220
CAS No.:9087-70-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 769169-27-9 | SDF | Download SDF |
| PubChem ID | 11269353 | Appearance | Powder |
| Formula | C9H8ClF6NO3S2 | M.Wt | 391.74 |
| Type of Compound | N/A | Storage | Desiccate at -20┬░C |
| Synonyms | GSI-953 | ||
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
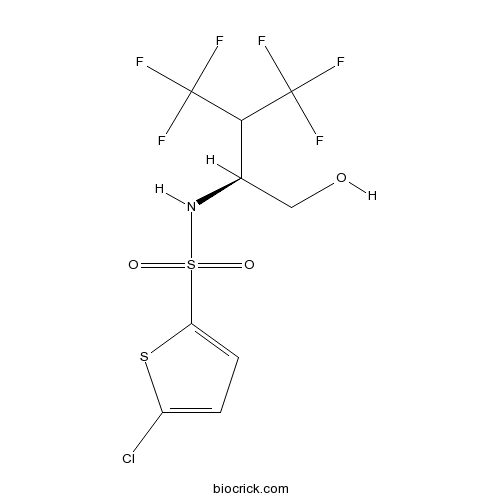
| Chemical Name | 5-chloro-N-[(2S)-4,4,4-trifluoro-1-hydroxy-3-(trifluoromethyl)butan-2-yl]thiophene-2-sulfonamide | ||
| SMILES | C1=C(SC(=C1)Cl)S(=O)(=O)NC(CO)C(C(F)(F)F)C(F)(F)F | ||
| Standard InChIKey | PSXOKXJMVRSARX-SCSAIBSYSA-N | ||
| Standard InChI | InChI=1S/C9H8ClF6NO3S2/c10-5-1-2-6(21-5)22(19,20)17-4(3-18)7(8(11,12)13)9(14,15)16/h1-2,4,7,17-18H,3H2/t4-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 Ōäā and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20Ōäā for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20Ōäā. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | γ-secretase inhibitor; selectively inhibits cleavage of amyloid precursor protein (APP) over Notch. Lowers levels of Aβ42 and Aβ40 (EC50 values are 12.4 and 14.8 nM respectively in cells expressing human recombinant APP). Orally active. |

Begacestat Dilution Calculator

Begacestat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5527 mL | 12.7636 mL | 25.5271 mL | 51.0543 mL | 63.8178 mL |
| 5 mM | 0.5105 mL | 2.5527 mL | 5.1054 mL | 10.2109 mL | 12.7636 mL |
| 10 mM | 0.2553 mL | 1.2764 mL | 2.5527 mL | 5.1054 mL | 6.3818 mL |
| 50 mM | 0.0511 mL | 0.2553 mL | 0.5105 mL | 1.0211 mL | 1.2764 mL |
| 100 mM | 0.0255 mL | 0.1276 mL | 0.2553 mL | 0.5105 mL | 0.6382 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
╬│-secretase inhibitor; selectively inhibits cleavage of amyloid precursor protein (APP) over Notch. Lowers levels of A╬▓42 and A╬▓40 (EC50 values are 12.4 and 14.8 nM respectively in cells expressing human recombinant APP). Orally active.
- 13-Methyl-8,11,13-podocarpatriene-3,12-diol
Catalog No.:BCN1360
CAS No.:769140-74-1
- Triacsin C
Catalog No.:BCC7377
CAS No.:76896-80-5
- Bz-Gly-OH.HCl
Catalog No.:BCC2945
CAS No.:7689-50-1
- Camptothecin
Catalog No.:BCN4318
CAS No.:7689-03-4
- Olivil 4'-O-glucoside
Catalog No.:BCN7557
CAS No.:76880-93-8
- 8-Methyl-8-azabicyclo[3.2.1]octane-3,6-diol, 9CI; (3RS,6RS)-form, 3-O-Ac
Catalog No.:BCN1361
CAS No.:7688-76-8
- 6-Hydroxywogonin
Catalog No.:BCN6556
CAS No.:76844-70-7
- Przewaquinone A
Catalog No.:BCN3004
CAS No.:76843-23-7
- 5-BDBD
Catalog No.:BCC7717
CAS No.:768404-03-1
- Famotidine
Catalog No.:BCC4529
CAS No.:76824-35-6
- Danshensu
Catalog No.:BCN8513
CAS No.:76822-21-4
- Pimaricin
Catalog No.:BCN2216
CAS No.:7681-93-8
- Viscosalactone B
Catalog No.:BCN7945
CAS No.:76938-46-0
- 8-Bromo-cAMP, sodium salt
Catalog No.:BCC8078
CAS No.:76939-46-3
- Onitin 2'-O-glucoside
Catalog No.:BCN4319
CAS No.:76947-60-9
- Cleomiscosin A
Catalog No.:BCN4320
CAS No.:76948-72-6
- DL-alpha-Tocopherylacetate
Catalog No.:BCN2904
CAS No.:7695-91-2
- Kalii Dehydrographolidi Succinas
Catalog No.:BCN8523
CAS No.:76958-99-1
- Nizatidine
Catalog No.:BCC4522
CAS No.:76963-41-2
- H-D-2-Nal-OH.HCl
Catalog No.:BCC3286
CAS No.:76985-09-6
- Boc-D-2-Nal-OH
Catalog No.:BCC3288
CAS No.:76985-10-9
- Cleomiscosin B
Catalog No.:BCN3898
CAS No.:76985-93-8
- TPT-260
Catalog No.:BCC5171
CAS No.:769856-81-7
- 15-Methoxypinusolidic acid
Catalog No.:BCN4321
CAS No.:769928-72-5
Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer's disease.[Pubmed:19671883]
J Pharmacol Exp Ther. 2009 Nov;331(2):598-608.
The presenilin containing gamma-secretase complex is responsible for the regulated intramembraneous proteolysis of the amyloid precursor protein (APP), the Notch receptor, and a multitude of other substrates. gamma-Secretase catalyzes the final step in the generation of Abeta(40) and Abeta(42) peptides from APP. Amyloid beta-peptides (Abeta peptides) aggregate to form neurotoxic oligomers, senile plaques, and congophilic angiopathy, some of the cardinal pathologies associated with Alzheimer's disease. Although inhibition of this protease acting on APP may result in potentially therapeutic reductions of neurotoxic Abeta peptides, nonselective inhibition of the enzyme may cause severe adverse events as a result of impaired Notch receptor processing. Here, we report the preclinical pharmacological profile of GSI-953 (Begacestat), a novel thiophene sulfonamide gamma-secretase inhibitor (GSI) that selectively inhibits cleavage of APP over Notch. This GSI inhibits Abeta production with low nanomolar potency in cellular and cell-free assays of gamma-secretase function, and displaces a tritiated analog of GSI-953 from enriched gamma-secretase enzyme complexes with similar potency. Cellular assays of Notch cleavage reveal that this compound is approximately 16-fold selective for the inhibition of APP cleavage. In the human APP-overexpressing Tg2576 transgenic mouse, treatment with this orally active compound results in a robust reduction in brain, plasma, and cerebral spinal fluid Abeta levels, and a reversal of contextual fear-conditioning deficits that are correlated with Abeta load. In healthy human volunteers, oral administration of a single dose of GSI-953 produces dose-dependent changes in plasma Abeta levels, confirming pharmacodynamic activity of GSI-953 in humans.
Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer's disease.[Pubmed:19012391]
J Med Chem. 2008 Dec 11;51(23):7348-51.
SAR on HTS hits 1 and 2 led to the potent, Notch-1-sparing GSI 9, which lowered brain Abeta in Tg2576 mice at 100 mg/kg po. Converting the metabolically labile methyl groups in 9 to trifluoromethyl groups afforded the more stable analogue 10, which had improved in vivo potency. Further side chain modification afforded the potent Notch-1-sparing GSI Begacestat (5), which was selected for development for the treatment of Alzheimer's disease.
ACS chemical neuroscience molecule spotlight on Begacestat (GSI-953).[Pubmed:22860177]
ACS Chem Neurosci. 2012 Jan 18;3(1):3-4.
A "second generation" gamma-secretase, Begacestat (GSI-953), which is more selective against Notch-signaling, has shown promise in recent Phase I clinical trials. Begacestat, a novel, 2,5-disubsitituted thiophene sulfonamide from Wyeth (now Pfizer) is under evaluation for the treatment of Alzheimer's disease.


