NizatidineCAS# 76963-41-2 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 76963-41-2 | SDF | Download SDF |
| PubChem ID | 3033637 | Appearance | Powder |
| Formula | C12H21N5O2S2 | M.Wt | 331.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (150.85 mM) H2O : 20 mg/mL (60.34 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
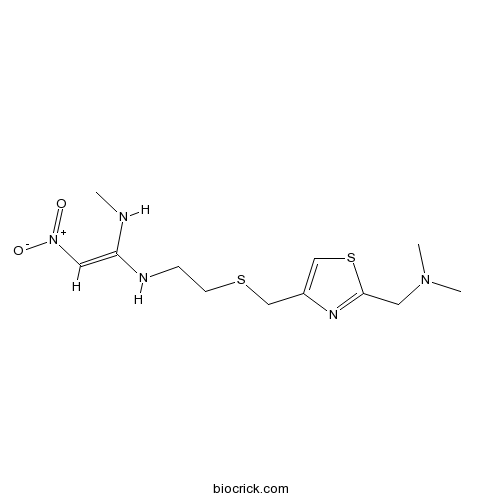
| Chemical Name | (E)-1-N'-[2-[[2-[(dimethylamino)methyl]-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine | ||
| SMILES | CNC(=C[N+](=O)[O-])NCCSCC1=CSC(=N1)CN(C)C | ||
| Standard InChIKey | SGXXNSQHWDMGGP-IZZDOVSWSA-N | ||
| Standard InChI | InChI=1S/C12H21N5O2S2/c1-13-11(6-17(18)19)14-4-5-20-8-10-9-21-12(15-10)7-16(2)3/h6,9,13-14H,4-5,7-8H2,1-3H3/b11-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nizatidine Dilution Calculator

Nizatidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.017 mL | 15.0848 mL | 30.1696 mL | 60.3391 mL | 75.4239 mL |
| 5 mM | 0.6034 mL | 3.017 mL | 6.0339 mL | 12.0678 mL | 15.0848 mL |
| 10 mM | 0.3017 mL | 1.5085 mL | 3.017 mL | 6.0339 mL | 7.5424 mL |
| 50 mM | 0.0603 mL | 0.3017 mL | 0.6034 mL | 1.2068 mL | 1.5085 mL |
| 100 mM | 0.0302 mL | 0.1508 mL | 0.3017 mL | 0.6034 mL | 0.7542 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nizatidine is a histamine H2 receptor antagonist with IC50 of 0.9 nM, also inhibits AChE with IC50 of 6.7 μM.
- Kalii Dehydrographolidi Succinas
Catalog No.:BCN8523
CAS No.:76958-99-1
- DL-alpha-Tocopherylacetate
Catalog No.:BCN2904
CAS No.:7695-91-2
- Cleomiscosin A
Catalog No.:BCN4320
CAS No.:76948-72-6
- Onitin 2'-O-glucoside
Catalog No.:BCN4319
CAS No.:76947-60-9
- 8-Bromo-cAMP, sodium salt
Catalog No.:BCC8078
CAS No.:76939-46-3
- Viscosalactone B
Catalog No.:BCN7945
CAS No.:76938-46-0
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
- 13-Methyl-8,11,13-podocarpatriene-3,12-diol
Catalog No.:BCN1360
CAS No.:769140-74-1
- Triacsin C
Catalog No.:BCC7377
CAS No.:76896-80-5
- Bz-Gly-OH.HCl
Catalog No.:BCC2945
CAS No.:7689-50-1
- Camptothecin
Catalog No.:BCN4318
CAS No.:7689-03-4
- Olivil 4'-O-glucoside
Catalog No.:BCN7557
CAS No.:76880-93-8
- H-D-2-Nal-OH.HCl
Catalog No.:BCC3286
CAS No.:76985-09-6
- Boc-D-2-Nal-OH
Catalog No.:BCC3288
CAS No.:76985-10-9
- Cleomiscosin B
Catalog No.:BCN3898
CAS No.:76985-93-8
- TPT-260
Catalog No.:BCC5171
CAS No.:769856-81-7
- 15-Methoxypinusolidic acid
Catalog No.:BCN4321
CAS No.:769928-72-5
- Notoginsenoside T5
Catalog No.:BCN3727
CAS No.:769932-34-5
- Euphroside
Catalog No.:BCN6633
CAS No.:76994-07-5
- Garcinone C
Catalog No.:BCN4322
CAS No.:76996-27-5
- Gibberellins
Catalog No.:BCN2189
CAS No.:77-06-5
- Chlorthalidone
Catalog No.:BCC4649
CAS No.:77-36-1
- Ursolic acid
Catalog No.:BCN4327
CAS No.:77-52-1
- Cedrol
Catalog No.:BCN8340
CAS No.:77-53-2
Molecular mobility in the supercooled and glassy states of nizatidine and perphenazine.[Pubmed:27916696]
Eur J Pharm Sci. 2017 Mar 1;99:147-151.
The dielectric properties of two pharmaceuticals Nizatidine and perphenazine were investigated in the supercooled liquid and glassy states by broadband dielectric spectroscopy. Two relaxation processes were observed in both the pharmaceuticals. The relaxation process observed above the glass transition temperature is the structural alpha relaxation and below the glass transition temperature is the gamma relaxation of intramolecular origin. The Johari-Goldstein beta relaxation coming from the motion of the entire molecule is found to be hidden under the structural relaxation peak in both the pharmaceuticals.
The Slow Relaxation Dynamics in the Amorphous Pharmaceutical Drugs Cimetidine, Nizatidine, and Famotidine.[Pubmed:27773524]
J Pharm Sci. 2016 Dec;105(12):3573-3584.
The slow molecular mobility in the amorphous solid state of 3 active pharmaceutical drugs (cimetidine, Nizatidine, and famotidine) has been studied using differential scanning calorimetry and the 2 dielectric-related techniques of dielectric relaxation spectroscopy and thermally stimulated depolarization currents. The glass-forming ability, the glass stability, and the tendency for crystallization from the equilibrium melt were investigated by differential scanning calorimetry, which also provided the characterization of the main relaxation of the 3 glass formers. The chemical instability of famotidine at the melting temperature and above it prevented the preparation of the amorphous for dielectric studies. In contrast, for cimetidine and Nizatidine, the dielectric study yielded the main kinetic features of the alpha relaxation and of the secondary relaxations. According to the obtained results, Nizatidine displays the higher fragility index of the 3 studied glass-forming drugs. The thermally stimulated depolarization current technique has proved useful to identify the Johari-Goldstein relaxation and to measure taubetaJG in the amorphous solid state, that is, in a frequency range which is not easily accessible by dielectric relaxation spectroscopy.
The Effect of Nizatidine, a MATE2K Selective Inhibitor, on the Pharmacokinetics and Pharmacodynamics of Metformin in Healthy Volunteers.[Pubmed:26507723]
Clin Pharmacokinet. 2016 Apr;55(4):495-506.
BACKGROUND AND OBJECTIVES: In the proximal tubule, basic drugs are transported from the renal cells to the tubule lumen through the concerted action of the H(+)/organic cation antiporters, multidrug and toxin extrusion (MATE) 1 and MATE2K. Dual inhibitors of the MATE transporters have been shown to have a clinically relevant effect on the pharmacokinetics of concomitantly administered basic drugs. However, the clinical impact of selective renal organic cation transport inhibition on the pharmacokinetics and pharmacodynamics of basic drugs, such as metformin, is unknown. This study sought to identify a selective MATE2K inhibitor in vitro and to determine its clinical impact on the pharmacokinetics and pharmacodynamics of metformin in healthy subjects. METHODS: Strategic cell-based screening of 71 US Food and Drug Administration (FDA)-approved medications was conducted to identify selective inhibitors of renal organic cation transporters that are capable of inhibiting at clinically relevant concentrations. From this screen, Nizatidine was identified and predicted to be a clinically potent and selective inhibitor of MATE2K-mediated transport. The effect of Nizatidine on the pharmacokinetics and pharmacodynamics of metformin was evaluated in 12 healthy volunteers in an open-label, randomized, two-phase crossover drug-drug interaction (DDI) study. RESULTS: In healthy volunteers, the MATE2K-selective inhibitor Nizatidine significantly increased the apparent volume of distribution, half-life, and hypoglycemic activity of metformin. However, despite achieving unbound maximum concentrations greater than the in vitro inhibition potency (concentration of drug producing 50% inhibition [IC50]) of MATE2K-mediated transport, Nizatidine did not affect the renal clearance (CLR) or net secretory clearance of metformin. CONCLUSION: This study demonstrates that a selective inhibition of MATE2K by Nizatidine affected the apparent volume of distribution, tissue concentrations, and peripheral effects of metformin. However, Nizatidine did not alter systemic concentrations or the CLR of metformin, suggesting that specific MATE2K inhibition may not be sufficient to cause renal DDIs with metformin.
Development and validation of a sensitive LC-MS/MS assay for the quantification of nizatidine in human plasma and urine and its application to pharmacokinetic study.[Pubmed:26197435]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Aug 15;998-999:80-7.
We developed and validated a high performance liquid chromatographic method coupled with triple quadrupole mass spectrometry for analysis of Nizatidine in human plasma and urine. The biological samples were precipitated with methanol before separation on an Agilent Eclipse Plus C18 column (100mmx46mm, 5mum) with a mixture of methanol and water (95:5, plus 5mM ammonium formate) as the mobile phase at 0.5mL/min. Detection was performed using multiple reaction monitoring modes via electrospray ionization (ESI) at m/z 332.1-->155.1 (for Nizatidine) and m/z 335.1-->155.1 (for [(2)H3]-Nizatidine, the internal standard). The linear response range was 5-2000ng/mL and 0.5-80mug/mL for human plasma and urine, with the lower limits of quantification of 5ng/mL and 0.5mug/mL, respectively. The method was validated according to the biological method validation guidelines of the Food and Drug Administration and proved acceptable. This newly developed analytical method was successfully applied in a pharmacokinetic study following single oral administration of a 150mg Nizatidine capsule in to 16 healthy Chinese subjects. Maximum and endpoint concentrations in plasma and urine were quantifiable, suggesting our method is appropriate for routine pharmacokinetic analysis.


