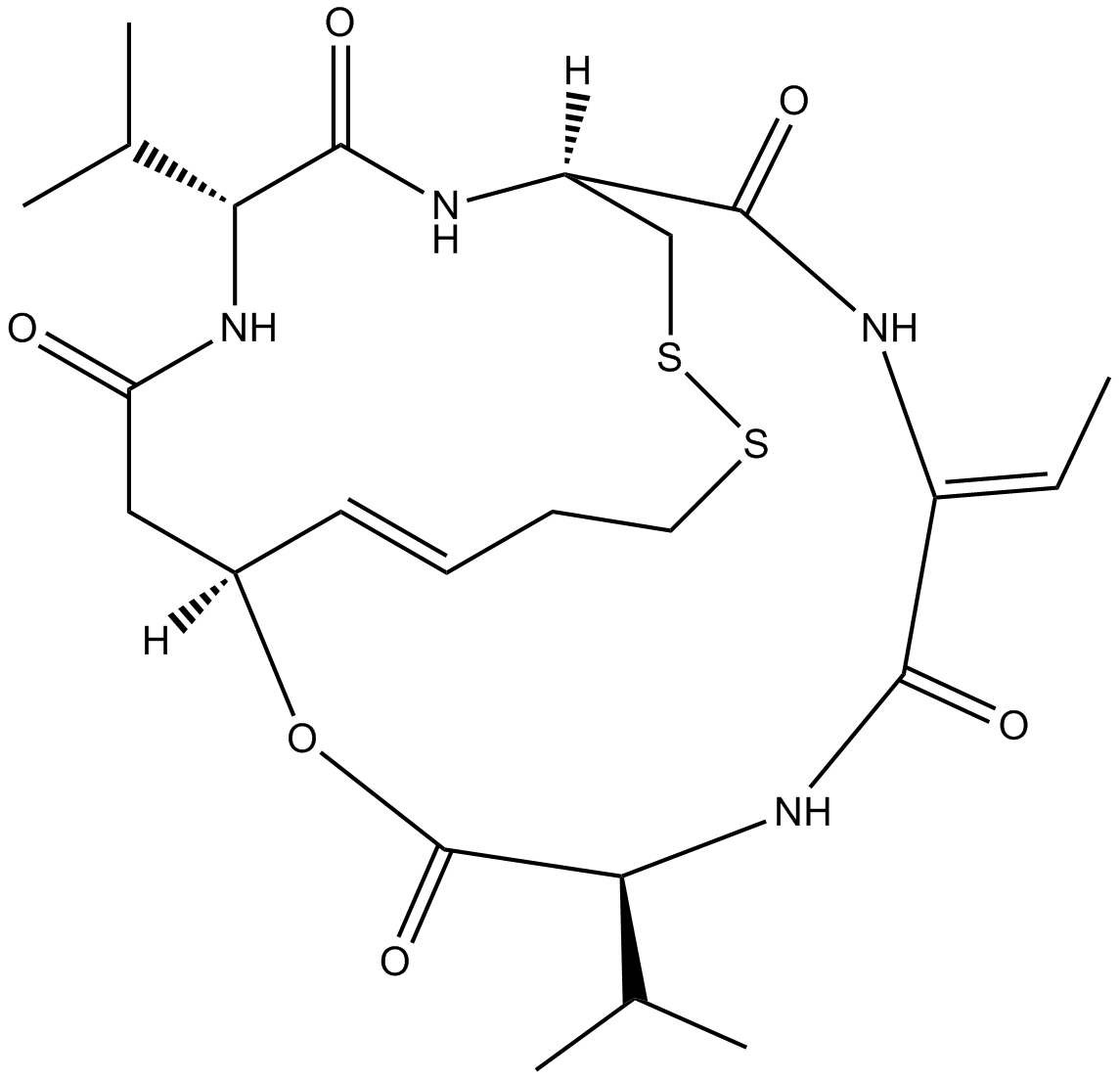
Romidepsin (FK228, depsipeptide)HDAC1/HDAC2 inhibitor,potent and selective CAS# 128517-07-7 |
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128517-07-7 | SDF | Download SDF |
| PubChem ID | 5352062 | Appearance | Powder |
| Formula | C24H36N4O6S2 | M.Wt | 540.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Istodax, Antibiotic FR 901228, FK228, FR 901228, FK-228,Romidepsin | ||
| Solubility | Soluble to 10 mM in DMSO | ||
| Chemical Name | (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone | ||
| SMILES | CC=C1C(=O)NC(C(=O)OC2CC(=O)NC(C(=O)NC(CSSCCC=C2)C(=O)N1)C(C)C)C(C)C | ||
| Standard InChIKey | OHRURASPPZQGQM-GCCNXGTGSA-N | ||
| Standard InChI | InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of class I histone deacetylases (HDACs) (IC50 values are 36, 47, 510 and 14,000 nM for HDAC1, HDAC2, HDAC4 and HDAC6 respectively). Exhibits cytotoxicity against various human tumor cell lines; also exhibits antitumor activity against human tumor xenografts. |

Romidepsin (FK228, depsipeptide) Dilution Calculator

Romidepsin (FK228, depsipeptide) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Romidepsin, also known as FK228 or depsipeptide, is potent and selective inhibitor of histone deacetylases (HDACs) which are associated with the regulation of re-expression of silenced tumor suppressor genes. It was the first HDAC inhibitor to manifest anti-tumor activity and originally isolated from a rod-shaped Gram-negative bacterium, Chromobacterium violaceum, found in a Japanese soil sample. Romidepsin exhibits a stronger inhibition towards HDAC1 and HDAC2 enzymes (class I), removing acetyl groups from the lysine residues of N-terminal histone tails and maintaining a more open and transcriptionally active chromatin state, than HDAC4 and HDAC6 enzymes (class II). Besides HDAC inhibition, romidepsin is also able to induce cell cycle arrest, cellular differentiation, apoptosis and alteration of gene expression in adult malignancies.
Reference
Karen M VanderMolen, William McCulloch, Cedric J Pearce and Nicholas H Oberlies. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma. The Journal of Antibiotics 2011: 64, 525-531
Jyoti Panicker, Zhijie Li, Christine McMahon, Caroline Sizer, Kenneth Steadman, Richard Piekarz, Susan E. Bates and Carol J. Thiele. Romidepsin (FK228/depsipeptide) controls growth and induces apoptosis in neuroblastoma tumor cells. Cell Cycle 2010 9:9, 1830-1838
- Ophiogenin-3-O-alpha-L-rhaMnopyranosyl-(1→2)-beta-D-glucopyranoside
Catalog No.:BCN1587
CAS No.:128502-94-3
- Methylophioponanone B
Catalog No.:BCN6525
CAS No.:128446-36-6
- 2-Hydroxypropyl-β-cyclodextrin
Catalog No.:BCC6757
CAS No.:128446-35-5
- Gelidoside
Catalog No.:BCN7320
CAS No.:128420-44-0
- Euojaponine D
Catalog No.:BCC8980
CAS No.:128397-42-2
- Hydroprotopine
Catalog No.:BCN6155
CAS No.:128397-41-1
- Hyptadienic acid
Catalog No.:BCN6154
CAS No.:128397-09-1
- MCH (human, mouse, rat)
Catalog No.:BCC6068
CAS No.:128315-56-0
- Cinalukast
Catalog No.:BCC7244
CAS No.:128312-51-6
- MK-8033 hydrochloride
Catalog No.:BCC4040
CAS No.:1283000-43-0
- 2alpha-Hydroxy-8beta-(2-methylbutyryloxy)costunolide
Catalog No.:BCN7319
CAS No.:128286-87-3
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- GSK2578215A
Catalog No.:BCC6243
CAS No.:1285515-21-0
- ML167
Catalog No.:BCC5348
CAS No.:1285702-20-6
- Pingpeimine C
Catalog No.:BCN8411
CAS No.:128585-96-6
- Ospemifene
Catalog No.:BCC5557
CAS No.:128607-22-7
- Z(2-Br)-Osu
Catalog No.:BCC2806
CAS No.:128611-93-8
- PyBOP
Catalog No.:BCC2820
CAS No.:128625-52-5
- 1,6-O,O-Diacetylbritannilactone
Catalog No.:BCN7792
CAS No.:1286694-67-4
- FRAX597
Catalog No.:BCC4172
CAS No.:1286739-19-2
- Eucamalduside A
Catalog No.:BCN7321
CAS No.:1287220-29-4
- Kalopanaxsaponin H
Catalog No.:BCN2572
CAS No.:128730-82-5
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- Fmoc-D-Asp(OtBu)-OH
Catalog No.:BCC3471
CAS No.:12883-39-3
Romidepsin (FK228/depsipeptide) controls growth and induces apoptosis in neuroblastoma tumor cells.[Pubmed:20404560]
Cell Cycle. 2010 May;9(9):1830-8.
As histone deacetylase inhibitors such as romidepsin (depsipeptide, FK228) complete successful Phase I clinical trials in pediatric solid tumors, it is important that their mechanisms of action are delineated in order to inform the development of subsequent clinical trials as single agents or in combination therapies. In this study, we evaluate the effect of romidepsin as a single agent on a number of different neuroblastoma (NB) cell lines. We find that the growth of 6/6 human NB tumor cell lines but not an immortalized fibroblast cell line (NIH3T3) is inhibited by romidepsin (IC(50) = 1-6.5 ng/ml) after 72 h of treatment. Romidepsin shows selective dose-dependent cytotoxicity in both single copy and N-myc amplified NB cell lines, in cell lines with wild type or mutant p53 and those containing Alk mutations. The decrease in cell proliferation is accompanied by caspase-dependent apoptosis as shown by PARP cleavage, an accumulation of cells in the sub-G(1) phase of the cell cycle and the ability of a pan-caspase inhibitor to reduce cell death. Romidepsin inhibits the growth of subcutaneous NB xenografts in a dose dependent manner in immunocompromised mice. Furthermore, romidepsin induces expression of genes such as p21 and expression of p75 and NTRK (TrkA) which are more highly expressed in the tumors from NB patients that have a good prognosis. These studies support continued investigations into the therapeutic activity of romidepsin in NB.
Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma.[Pubmed:21587264]
J Antibiot (Tokyo). 2011 Aug;64(8):525-31.
Romidepsin (Istodax), a selective inhibitor of histone deacetylases (HDACs), was approved for the treatment of cutaneous T-cell lymphoma in November 2009 by the US Food and Drug Administration. This unique natural product was discovered from cultures of Chromobacterium violaceum, a Gram-negative bacterium isolated from a Japanese soil sample. This bicyclic compound acts as a prodrug, its disulfide bridge being reduced by glutathione on uptake into the cell, allowing the free thiol groups to interact with Zn ions in the active site of class I and II HDAC enzymes. Due to the synthetic complexity of the compound, as well as the low yield from the producing organism, analogs are sought to create synthetically accessible alternatives. As a T-cell lymphoma drug, romidepsin offers a valuable new treatment for diseases with few effective therapies.
Involvement of extracellular signal-regulated kinase activation in human osteosarcoma cell resistance to the histone deacetylase inhibitor FK228 [(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dithia-5,8 ,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone].[Pubmed:19073909]
J Pharmacol Exp Ther. 2009 Mar;328(3):839-48.
The histone deacetylase inhibitor depsipeptide [(1S,4S,7Z,10S, 16E,21R)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraaza bicyclo[8.7.6]tricos-16-ene-3,6,9,19, 22-pentone] (FK228) has attracted a great deal of interest because of its antiproliferative and apoptotic properties in various malignancies. Histone deacetylase inhibitors induce the expression of the multidrug resistance transporter P-glycoprotein (P-gp), and FK228 is a known P-gp substrate. Thus, FK228 seems to induce its own mechanism of drug resistance by up-regulating P-gp. The goal of this study was to establish human FK228-resistant osteosarcoma cell lines and to investigate whether there are mechanisms of FK228 resistance in addition to P-gp up-regulation. After 72 h in culture, the 50% inhibitory concentrations (IC(50)) of FK228 were 4.8 and 991 nM in HOS and HOS/FK8 cells, respectively, and 3.6 and 1420 nM in U2OS and U2OS/FK11 cells, respectively. Increased histone H3 acetylation was observed in FK228-resistant cell lines after a 1-h treatment with 10 nM FK228. Unlike in parental cells, significant P-gp overexpression was detected in FK228-resistant cells, and 10 nM FK228 treatment activated the mitogen-activated protein kinase (MAPK) pathway but did not induce Fas ligand (FasL) up-regulation or c-FLIP down-regulation. However, treatment of FK228-resistant cells with a combination of FK228 and mitogen-activated protein kinase kinase (MEK) inhibitors induced apoptosis, up-regulated FasL, and down-regulated c-FLIP. The expression and function of P-gp were unaltered by treatment with MEK inhibitors. These results indicate that the FK228 resistance of osteosarcoma cells is related to P-gp overexpression and MAPK pathway activation by FK228. MEK or P-gp inhibitors may be useful in overcoming this resistance.
Effects of FK228, a novel histone deacetylase inhibitor, on tumor growth and expression of p21 and c-myc genes in vivo.[Pubmed:12767524]
Cancer Lett. 2003 Jun 10;195(2):161-8.
In this study, we examined the effects of FK228 (FR901228, depsipeptide) on tumor growth and expression of p21 and c-myc genes in vivo. FK228 induced the expression of p21 mRNA and decreased c-myc mRNA in tumor xenograft sensitive to FK228. However, FK228 did not sufficiently modulate the expression of p21 mRNA and increased the expression of c-myc in tumor xenograft less sensitive to FK228. The modulation of p21 and/or c-myc genes may be critical for the marked antitumor activity of FK228 in vivo.
FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases.[Pubmed:12208741]
Cancer Res. 2002 Sep 1;62(17):4916-21.
FK228 is a histone deacetylase (HDAC) inhibitor, the molecular mechanism of inhibition of which has been unknown. Here we show that reduction of an intramolecular disulfide bond of FK228 greatly enhanced its inhibitory activity and that the disulfide bond was rapidly reduced in cells by cellular reducing activity involving glutathione. Computer modeling suggests that one of the sulfhydryl groups of the reduced form of FK228 (redFK) interacts with the active-site zinc, preventing the access of the substrate. HDAC1 and HDAC2 were more strongly inhibited by redFK than HDAC4 and HDAC6. redFK was less active than FK228 in inhibiting in vivo HDAC activity, due to rapid inactivation in medium and serum. Thus, FK228 serves as a stable prodrug to inhibit class I enzymes and is activated by reduction after uptake into the cells. The glutathione-mediated activation also implicates its clinical usefulness for counteracting glutathione-mediated drug resistance in chemotherapy.


