Bivalirudin TrifluoroacetateReversible thrombin inhibitor CAS# 128270-60-0 |
- Dabigatran ethyl ester
Catalog No.:BCC1512
CAS No.:429658-95-7
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128270-60-0 | SDF | Download SDF |
| PubChem ID | 16129704 | Appearance | Powder |
| Formula | C98H137DN24O33 | M.Wt | 2181.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Bivalirudin | ||
| Solubility | DMSO : ≥ 31 mg/mL (13.51 mM); | ||
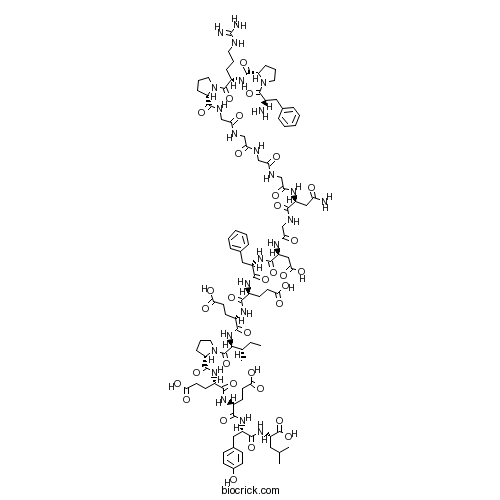
| SMILES | CCC(C)C(C(=O)N1CCCC1C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(CC2=CC=C(C=C2)O)C(=O)NC(CC(C)C)C(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CC3=CC=CC=C3)NC(=O)C(CC(=O)O)NC(=O)CNC(=O)C(CC(=O)N)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)C4CCCN4C(=O)C(CCCNC(=N)N)NC(=O)C5CCCN5C(=O)C(CC6=CC=CC=C6)N | ||
| Standard InChIKey | OIRCOABEOLEUMC-GEJPAHFPSA-N | ||
| Standard InChI | InChI=1S/C98H138N24O33/c1-5-52(4)82(96(153)122-39-15-23-70(122)92(149)114-60(30-34-79(134)135)85(142)111-59(29-33-78(132)133)86(143)116-64(43-55-24-26-56(123)27-25-55)89(146)118-67(97(154)155)40-51(2)3)119-87(144)61(31-35-80(136)137)112-84(141)58(28-32-77(130)131)113-88(145)63(42-54-18-10-7-11-19-54)117-90(147)66(45-81(138)139)110-76(129)50-107-83(140)65(44-71(100)124)109-75(128)49-106-73(126)47-104-72(125)46-105-74(127)48-108-91(148)68-21-13-38-121(68)95(152)62(20-12-36-103-98(101)102)115-93(150)69-22-14-37-120(69)94(151)57(99)41-53-16-8-6-9-17-53/h6-11,16-19,24-27,51-52,57-70,82,123H,5,12-15,20-23,28-50,99H2,1-4H3,(H2,100,124)(H,104,125)(H,105,127)(H,106,126)(H,107,140)(H,108,148)(H,109,128)(H,110,129)(H,111,142)(H,112,141)(H,113,145)(H,114,149)(H,115,150)(H,116,143)(H,117,147)(H,118,146)(H,119,144)(H,130,131)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,154,155)(H4,101,102,103)/t52-,57+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,82-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bivalirudin Trifluoroacetate is a synthetic 20 residue peptide which reversibly inhibits thrombin.
IC50 Value:
Target: thrombin
in vitro: Eptifibatide (8 mg/mL) added together with a low (70 ng/mL) concentration of bivalirudin (a direct thrombin inhibitor) effectively (approximately 90%) reduced platelet aggregation induced by thrombin (0.2 U/mL) [1]. In thrombin generation assay (TGA), bivalirudin had no effect on these parameters up to 10 μmol/l [2]. Bivalirudin-facilitated binding of MPO to BAEC resulted also in functional changes in terms of increased NO consumption as well as enhanced MPO-mediated redox modifications [3].
in vivo: The use of bivalirudinprevented further increase in antiheparin/PF4 antibody IgG levels in rats [4]. Three animals in the 500-mg/kg/24 h group, and 7 animals in the 2000-mg/kg/24 h group in the toxicokinetic assessment phase of the study were found dead or euthanized in extremis (following blood sampling). Plasma concentrations of bivalirudin appeared to be linear and dose independent [5].
Clinical trial: Antithrombotic Effects of Ticagrelor Versus Clopidogrel . Phase 4 References: | |||||

Bivalirudin Trifluoroacetate Dilution Calculator

Bivalirudin Trifluoroacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.4584 mL | 2.2922 mL | 4.5844 mL | 9.1689 mL | 11.4611 mL |
| 5 mM | 0.0917 mL | 0.4584 mL | 0.9169 mL | 1.8338 mL | 2.2922 mL |
| 10 mM | 0.0458 mL | 0.2292 mL | 0.4584 mL | 0.9169 mL | 1.1461 mL |
| 50 mM | 0.0092 mL | 0.0458 mL | 0.0917 mL | 0.1834 mL | 0.2292 mL |
| 100 mM | 0.0046 mL | 0.0229 mL | 0.0458 mL | 0.0917 mL | 0.1146 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Bivalirudin is a synthetic 20 residue peptide (thrombin inhibitor) which reversibly inhibits thrombin. in vitro: Eptifibatide (8 mg/mL) added together with a low (70 ng/mL) concentration of bivalirudin (a direct thrombin inhibitor) effectively (approximately 90%) reduced platelet aggregation induced by thrombin (0.2 U/mL) [1]. In thrombin generation assay (TGA), bivalirudin had no effect on these parameters up to 10 μmol/l [2]. Bivalirudin-facilitated binding of MPO to BAEC resulted also in functional changes in terms of increased NO consumption as well as enhanced MPO-mediated redox modifications [3]. in vivo: The use of bivalirudinprevented further increase in antiheparin/PF4 antibody IgG levels in rats [4]. Three animals in the 500-mg/kg/24 h group, and 7 animals in the 2000-mg/kg/24 h group in the toxicokinetic assessment phase of the study were found dead or euthanized in extremis (following blood sampling). Plasma concentrations of bivalirudin appeared to be linear and dose independent [5]. Clinical trial: Antithrombotic Effects of Ticagrelor Versus Clopidogrel . Phase 4
- 1-(3,4-Dimethoxycinnamoyl)piperidine
Catalog No.:BCN4036
CAS No.:128261-84-7
- Axillaridine A
Catalog No.:BCN6153
CAS No.:128255-16-3
- Pachyaximine A
Catalog No.:BCN6152
CAS No.:128255-08-3
- BAY-X 1005
Catalog No.:BCC6038
CAS No.:128253-31-6
- GDC-0032
Catalog No.:BCC4066
CAS No.:1282512-48-4
- (R,R)-2,6-Bis(4-phenyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8397
CAS No.:128249-70-7
- N-ArachidonylGABA
Catalog No.:BCC7186
CAS No.:128201-89-8
- Escitalopram
Catalog No.:BCC4193
CAS No.:128196-01-0
- Fmoc-D-Ser(tBu)-OH
Catalog No.:BCC3548
CAS No.:128107-47-1
- erythro-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1588
CAS No.:1280602-81-4
- Arvanil
Catalog No.:BCC7026
CAS No.:128007-31-8
- Sennoside B
Catalog No.:BCN1003
CAS No.:128-57-4
- 2alpha-Hydroxy-8beta-(2-methylbutyryloxy)costunolide
Catalog No.:BCN7319
CAS No.:128286-87-3
- MK-8033 hydrochloride
Catalog No.:BCC4040
CAS No.:1283000-43-0
- Cinalukast
Catalog No.:BCC7244
CAS No.:128312-51-6
- MCH (human, mouse, rat)
Catalog No.:BCC6068
CAS No.:128315-56-0
- Hyptadienic acid
Catalog No.:BCN6154
CAS No.:128397-09-1
- Hydroprotopine
Catalog No.:BCN6155
CAS No.:128397-41-1
- Euojaponine D
Catalog No.:BCC8980
CAS No.:128397-42-2
- Gelidoside
Catalog No.:BCN7320
CAS No.:128420-44-0
- 2-Hydroxypropyl-β-cyclodextrin
Catalog No.:BCC6757
CAS No.:128446-35-5
- Methylophioponanone B
Catalog No.:BCN6525
CAS No.:128446-36-6
- Ophiogenin-3-O-alpha-L-rhaMnopyranosyl-(1→2)-beta-D-glucopyranoside
Catalog No.:BCN1587
CAS No.:128502-94-3
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
Liquid chromatography method for determination of bivalirudin in human plasma and urine using automated ortho-phthalaldehyde derivatization and fluorescence detection.[Pubmed:15018798]
J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Apr 5;802(2):355-9.
A high-performance liquid chromatographic (HPLC) method was developed using solid-phase extraction, o-phthalaldehyde (OPA) derivatization and fluorescence detection for the determination of the direct thrombin inhibitor bivalirudin in human plasma and urine. The use of this assay will facilitate the study of the pharmacodynamics of bivalirudin in studies of special patient populations. A C(18) bioanalytical column at a flow rate of 1 ml/min with an aqueous trifluoroacetic acid (0.1% TFA in deionized water, pH 2.2, v/v) mobile phase and methanol gradient was used. The assay demonstrated linearity from 3 to 20 microg/ml bivalirudin in plasma, with a detection limit of 1 microg/ml. The method was utilized in a study evaluating the pharmacokinetic and pharmacodynamic effects of bivalirudin in patients undergoing percutaneous coronary interventions (PCIs).


