Dabigatran etexilate mesylateDirect thrombin inhibitor,anticoagulant,prodrug of dabigatran CAS# 872728-81-9 |
Quality Control & MSDS
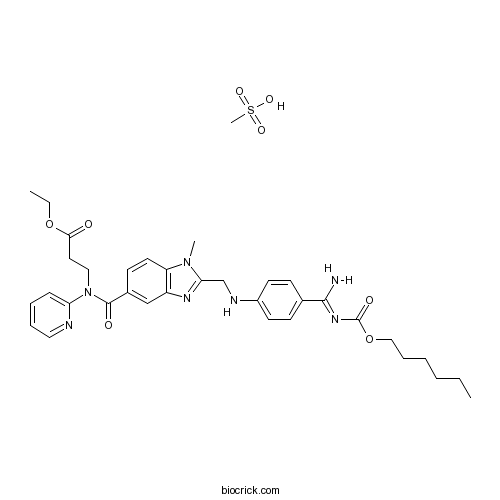
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 872728-81-9 | SDF | Download SDF |
| PubChem ID | 72960092 | Appearance | Powder |
| Formula | C35H45N7O8S | M.Wt | 723.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BIBR 1048MS; Dabigatran etexilate methanesulfonate | ||
| Solubility | DMSO : 50 mg/mL (69.08 mM; Need ultrasonic) | ||
| Chemical Name | ethyl 3-[[2-[[4-(N'-hexoxycarbonylcarbamimidoyl)anilino]methyl]-1-methylbenzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoate;methanesulfonic acid | ||
| SMILES | CCCCCCOC(=O)N=C(C1=CC=C(C=C1)NCC2=NC3=C(N2C)C=CC(=C3)C(=O)N(CCC(=O)OCC)C4=CC=CC=N4)N.CS(=O)(=O)O | ||
| Standard InChIKey | XETBXHPXHHOLOE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C34H41N7O5.CH4O3S/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29;1-5(2,3)4/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dabigatran etexilate mesylate (BIBR 1048MS) is the orally active prodrug of dabigatran. Dabigatran is a reversible and selective, direct thrombin inhibitor (DTI) with Ki value of 4.5 nM.
IC50 Value: 4.5 nM (Ki); 10 nM(Thrombin-induced platelet aggregation) [1]
in vitro: Dabigatran selectively and reversibly inhibited human thrombin(Ki: 4.5 nM) as well as thrombin-induced platelet aggregation (IC(50): 10 nM), while showing no inhibitory effect on other platelet-stimulating agents.Thrombin generation in platelet-poor plasma (PPP), measured as the endogenous thrombin potential (ETP) was inhibited concentration-dependently (IC(50): 0.56 microM). Dabigatran demonstrated concentration-dependent anticoagulant effects in various species in vitro, doubling the activated partial thromboplastin time (aPTT), prothrombin time (PT) and ecarin clotting time (ECT) in human PPP at concentrations of 0.23, 0.83 and 0.18 microM, respectively [1].
in vivo: Dabigatran prolonged the aPTT dose-dependently after intravenous administration in rats (0.3, 1 and 3 mg/kg) and rhesus monkeys (0.15, 0.3 and 0.6 mg/kg). Dose- and time-dependent anticoagulant effects were observed with dabigatran etexilate administered orally to conscious rats (10, 20 and 50 mg/kg) or rhesus monkeys (1, 2.5 or 5 mg/kg), with maximum effects observed between 30 and 120 min after administration, respectively [1]. Patients treated with dabigatran etexilate experienced fewer ischaemic strokes (3.74 dabigatran etexilate vs 3.97 warfarin) and fewer combined intracranial haemorrhages and haemorrhagic strokes (0.43 dabigatran etexilate vs 0.99 warfarin) per 100 patient-years [2].
Clinical trial: An Evaluation of the Pharmacokinetics and Pharmacodynamics of Oral Dabigatran Etexilate in Hemodialysis Patients . Phase1 References: | |||||

Dabigatran etexilate mesylate Dilution Calculator

Dabigatran etexilate mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3815 mL | 6.9076 mL | 13.8152 mL | 27.6304 mL | 34.538 mL |
| 5 mM | 0.2763 mL | 1.3815 mL | 2.763 mL | 5.5261 mL | 6.9076 mL |
| 10 mM | 0.1382 mL | 0.6908 mL | 1.3815 mL | 2.763 mL | 3.4538 mL |
| 50 mM | 0.0276 mL | 0.1382 mL | 0.2763 mL | 0.5526 mL | 0.6908 mL |
| 100 mM | 0.0138 mL | 0.0691 mL | 0.1382 mL | 0.2763 mL | 0.3454 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 4.5nM (Ki); 10nM(Thrombin-induced platelet aggregation) [1] Dabigatran is a reversible and selective, direct thrombin inhibitor (DTI) undergoing advanced clinical development as its orally active prodrug,dabigatran etexilate. in vitro: Dabigatran selectively and reversibly inhibited human thrombin(Ki: 4.5 nM) as well as thrombin-induced platelet aggregation (IC(50): 10 nM), while showing no inhibitory effect on other platelet-stimulating agents.Thrombin generation in platelet-poor plasma (PPP), measured as the endogenous thrombin potential (ETP) was inhibited concentration-dependently (IC(50): 0.56 microM). Dabigatran demonstrated concentration-dependent anticoagulant effects in various species in vitro, doubling the activated partial thromboplastin time (aPTT), prothrombin time (PT) and ecarin clotting time (ECT) in human PPP at concentrations of 0.23, 0.83 and 0.18 microM, respectively [1]. in vivo: Dabigatran prolonged the aPTT dose-dependently after intravenous administration in rats (0.3, 1 and 3 mg/kg) and rhesus monkeys (0.15, 0.3 and 0.6 mg/kg). Dose- and time-dependent anticoagulant effects were observed with dabigatran etexilate administered orally to conscious rats (10, 20 and 50 mg/kg) or rhesus monkeys (1, 2.5 or 5 mg/kg), with maximum effects observed between 30 and 120 min after administration, respectively [1]. Patients treated with dabigatran etexilate experienced fewer ischaemic strokes (3.74 dabigatran etexilate vs 3.97 warfarin) and fewer combined intracranial haemorrhages and haemorrhagic strokes (0.43 dabigatran etexilate vs 0.99 warfarin) per 100 patient-years [2]. Clinical trial: An Evaluation of the Pharmacokinetics and Pharmacodynamics of Oral Dabigatran Etexilate in Hemodialysis Patients . Phase1
- MEDICA 16
Catalog No.:BCC7956
CAS No.:87272-20-6
- 7-Epi-5-eudesmene-1beta,11-diol
Catalog No.:BCN7701
CAS No.:87261-77-6
- Ro 3306
Catalog No.:BCC4007
CAS No.:872573-93-8
- BGJ398
Catalog No.:BCC1278
CAS No.:872511-34-7
- (+)-Noe's reagent
Catalog No.:BCC8377
CAS No.:87248-50-8
- Bijaponicaxanthone C
Catalog No.:BCN6884
CAS No.:872409-35-3
- NVP-QAV680
Catalog No.:BCC5508
CAS No.:872365-16-7
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
- K 114
Catalog No.:BCC5984
CAS No.:872201-12-2
- Dihydrotanshinone I
Catalog No.:BCN4417
CAS No.:87205-99-0
- WAY 207024 dihydrochloride
Catalog No.:BCC7802
CAS No.:872002-73-8
- 2-Deacetoxydecinnamoyltaxinine J
Catalog No.:BCN7218
CAS No.:87193-98-4
- 1,2,3,10-Tetramethoxy-9-(2-hydroxy-4,5-dimethoxybenzyloxy)oxoaporphine
Catalog No.:BCN8120
CAS No.:872729-33-4
- 3-Methoxyoxohernandaline
Catalog No.:BCN8107
CAS No.:872729-34-5
- 6-Aminouracil
Catalog No.:BCC8768
CAS No.:873-83-6
- 3,29-Dibenzoyl karounitriol
Catalog No.:BCN2717
CAS No.:873001-54-8
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
- Ganoderone A
Catalog No.:BCN2448
CAS No.:873061-79-1
- IKK-16 (IKK Inhibitor VII)
Catalog No.:BCC4555
CAS No.:873225-46-8
- AIM-100
Catalog No.:BCC1333
CAS No.:873305-35-2
- Ramipril
Catalog No.:BCC5012
CAS No.:87333-19-5
- 1,7-Dihydroxy-4-methoxyxanthone
Catalog No.:BCN7602
CAS No.:87339-76-2
- TC-P 262
Catalog No.:BCC6155
CAS No.:873398-67-5
- Fortuneine
Catalog No.:BCN6401
CAS No.:87340-25-8
Effect of bosutinib on the absorption of dabigatran etexilate mesylate, a P-glycoprotein substrate, in healthy subjects.[Pubmed:27717999]
Eur J Clin Pharmacol. 2017 Jan;73(1):57-63.
PURPOSE: Bosutinib, a dual Src and Abl tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia, demonstrated concentration-dependent inhibitory effects on P-glycoprotein (P-gp)-mediated digoxin efflux in vitro, suggesting that bosutinib may inhibit P-gp substrates. The effect of bosutinib on Dabigatran etexilate mesylate (EM) absorption, a P-gp substrate, was evaluated. METHODS: In this open-label, randomized, single-dose, one-cohort, two-sequence, two-period crossover study, healthy, fed subjects received dabigatran EM (150 mg x 1 orally) alone or 1 h after receiving bosutinib tablets (100 mg x 5 orally). RESULTS: Dabigatran EM monotherapy and concurrent administration of dabigatran EM with bosutinib resulted in similar values for concentration time curves from time zero extrapolated to infinity (AUCinf), but slightly lower maximum plasma concentration (C max) values (AUCinf, 1182 and 1186 ng.h/mL, respectively; C max, 129.8 and 114.1 ng/mL). The time to maximum concentration for dabigatran was 2.99 and 3.99 h for combination therapy. The ratio of the adjusted geometric means (test/reference) of dabigatran AUCinf and C max (90 % confidence interval) were 101.4 % (89.6-114.9 %) and 89.7 % (77.8-103.4 %), respectively, following administration of dabigatran EM with bosutinib (test) relative to dabigatran EM administered alone (reference). Six subjects receiving combination treatment reported a total of seven adverse events (AEs) versus none for subjects receiving monotherapy alone. All AEs were mild to moderate and considered treatment related. CONCLUSION: These data demonstrate that single doses of bosutinib do not affect dabigatran exposure, suggesting that bosutinib is not a clinical inhibitor of P-gp. TRIAL REGISTRATION: ClinicalTrials.gov NCT02102633. https://clinicaltrials.gov/ct2/show/NCT02102633?term=NCT02102633&rank=1.
Transfer of dabigatran and dabigatran etexilate mesylate across the dually perfused human placenta.[Pubmed:24807346]
Obstet Gynecol. 2014 Jun;123(6):1256-61.
OBJECTIVE: To assess the transplacental pharmacokinetics at term of the oral thrombin inhibitor, dabigatran, and its prodrug, Dabigatran etexilate mesylate, to estimate fetal drug exposure. METHODS: Placentae were obtained with informed consent after cesarean delivery of healthy term pregnancies in Toronto, Ontario, Canada. The transplacental transfer of dabigatran and Dabigatran etexilate mesylate was separately assessed using the ex vivo dual perfusion of an isolated human placental cotyledon. Dabigatran, at a concentration of 35 ng/mL, was added to the maternal circulation at the start of the experimental phase. Maternal and fetal samples were taken throughout the preexperimental (1 hour) and experimental (3 hours) phases for measurement of dabigatran and markers of placental viability. Separate placenta perfusions with Dabigatran etexilate mesylate were conducted at an initial maternal concentration of 3.5 ng/mL. Dabigatran and Dabigatran etexilate mesylate were measured using liquid chromatography-tandem mass spectrometry. RESULTS: There was slower transfer of dabigatran compared with antipyrine from the maternal-to-fetal circulation, because the median fetal-to-maternal concentration ratio was 0.33 (interquartile range 0.29-0.38) after 3 hours (n=3). The prodrug, Dabigatran etexilate mesylate, had limited placental transfer as characterized by a fetal-to-maternal ratio of 0.17 (interquartile range 0.15-0.17) after 3 hours (n=3). Placental viability markers for all perfusions were within normal ranges. CONCLUSION: This report provides direct evidence of the transfer of dabigatran and its prodrug across the term human placenta from the mother to the fetus. From a clinical perspective, these data suggest that, pending further study, dabigatran should not be used for anticoagulation of pregnant women, because the drug may have an adverse effect on fetal blood coagulation.
Estimation Based on Emission Wavelength of Dabigatran Etexilate Mesylate in Bulk and Capsule Dosage Form.[Pubmed:27168697]
Indian J Pharm Sci. 2016 Jan-Feb;78(1):166-9.
A simple, rapid, specific and highly sensitive spectrofluorimetric method has been developed for the quantification of Dabigatran etexilate mesylate in bulk and capsule dosage form. A linear relationship was found between fluorescence intensity and concentration in the range of 0.01-1.0 mug/ml in dimethyl sulphoxide as solvent at an emission wavelength of 391 nm after excitation at 334 nm, with a good correlation coefficient (0.989). The detection and quantification limits were found to be 0.005 and 0.015 mug/ml, respectively. The proposed method was applied for Dabigatran etexilate mesylate capsules, results reveal with percentage recovery of 102% and percentage relative standard deviation values were found to be less than 2 for accuracy and precision studies. The proposed method was validated for linearity, range, accuracy, precision, limit of detection and quantification according to International Conference on Harmonization guidelines. Statistical analysis of the results revealed high accuracy and good precision. The suggested procedures could be used for the determination of Dabigatran etexilate mesylate in bulk and capsule dosage form in quality control laboratories of industries as well as in academic institutions.


