U-73122Phospholipase C inhibitor CAS# 112648-68-7 |
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Dorzolamide HCl
Catalog No.:BCC2311
CAS No.:130693-82-2
- Brinzolamide
Catalog No.:BCC2313
CAS No.:138890-62-7
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112648-68-7 | SDF | Download SDF |
| PubChem ID | 104794 | Appearance | Powder |
| Formula | C29H40N2O3 | M.Wt | 464.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 12.5 mg/mL (26.90 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
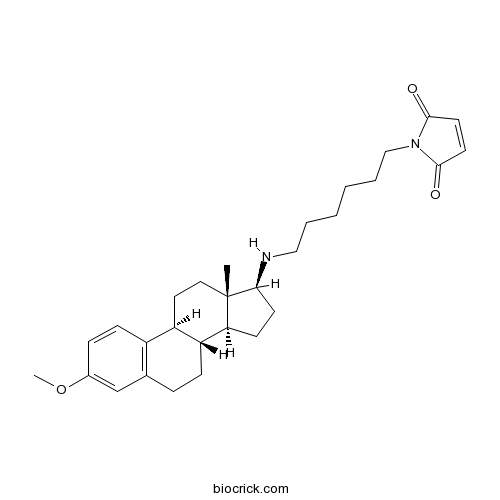
| Chemical Name | 1-[6-[[(8R,9S,13S,14S,17S)-3-methoxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]amino]hexyl]pyrrole-2,5-dione | ||
| SMILES | CC12CCC3C(C1CCC2NCCCCCCN4C(=O)C=CC4=O)CCC5=C3C=CC(=C5)OC | ||
| Standard InChIKey | LUFAORPFSVMJIW-ZRJUGLEFSA-N | ||
| Standard InChI | InChI=1S/C29H40N2O3/c1-29-16-15-23-22-10-8-21(34-2)19-20(22)7-9-24(23)25(29)11-12-26(29)30-17-5-3-4-6-18-31-27(32)13-14-28(31)33/h8,10,13-14,19,23-26,30H,3-7,9,11-12,15-18H2,1-2H3/t23-,24-,25+,26+,29+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Phospholipase C inhibitor. Inhibits agonist-induced platelet aggregation with IC50 values of 1-5 μM. Potently inhibits human polymorphonuclear neutrophil adhesion on biological surfaces (IC50 < 50 nM) and exhibits antinociceptive activity in vivo. Also activates TRPM4 and inhibits TRPM3 channels. Negative control U 73343 also available. |

U-73122 Dilution Calculator

U-73122 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1522 mL | 10.761 mL | 21.522 mL | 43.0441 mL | 53.8051 mL |
| 5 mM | 0.4304 mL | 2.1522 mL | 4.3044 mL | 8.6088 mL | 10.761 mL |
| 10 mM | 0.2152 mL | 1.0761 mL | 2.1522 mL | 4.3044 mL | 5.3805 mL |
| 50 mM | 0.043 mL | 0.2152 mL | 0.4304 mL | 0.8609 mL | 1.0761 mL |
| 100 mM | 0.0215 mL | 0.1076 mL | 0.2152 mL | 0.4304 mL | 0.5381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
U-73122 is an inhibitor of phospholipase C, phospholipase A2, and 5-LO (5-lipoxygenase).
- Dicyclanil
Catalog No.:BCC8938
CAS No.:112636-83-6
- Iso-mogroside V
Catalog No.:BCN3047
CAS No.:1126032-65-2
- 4-Allylpyrocatechol
Catalog No.:BCN6009
CAS No.:1126-61-0
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- A 804598
Catalog No.:BCC6198
CAS No.:1125758-85-1
- Mps1-IN-1
Catalog No.:BCC5590
CAS No.:1125593-20-5
- 4-O-Methylepisappanol
Catalog No.:BCN3674
CAS No.:112529-37-0
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- ent-16alpha,17-Dihydroxyatisan-3-one
Catalog No.:BCN6607
CAS No.:112523-91-8
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Citrusinol
Catalog No.:BCN8083
CAS No.:112516-43-5
- Tubuloside A
Catalog No.:BCN2806
CAS No.:112516-05-9
- Garcinone E
Catalog No.:BCN3604
CAS No.:112649-21-5
- BR-Xanthone A
Catalog No.:BCN6007
CAS No.:112649-48-6
- 1,7-Dihydroxyacridone
Catalog No.:BCN7275
CAS No.:112649-95-3
- Fragransin A2
Catalog No.:BCN6008
CAS No.:112652-46-7
- Erigeside C
Catalog No.:BCN6010
CAS No.:112667-09-1
- Oleonuezhenide
Catalog No.:BCN6011
CAS No.:112693-21-7
- IWR-1-endo
Catalog No.:BCC5102
CAS No.:1127442-82-3
- exo-IWR 1
Catalog No.:BCC7823
CAS No.:1127442-87-8
- Galanin (1-15) (porcine, rat)
Catalog No.:BCC5762
CAS No.:112747-70-3
- Clemastanin B
Catalog No.:BCC8152
CAS No.:112747-98-5
- Osthenone
Catalog No.:BCN4731
CAS No.:112789-90-9
- Letrozole
Catalog No.:BCC1063
CAS No.:112809-51-5
Imidazole-induced contractility of vascular smooth muscle cells in the presence of U-73122, ODQ, indomethacin and 7-nitroindazole.[Pubmed:23971197]
Pol J Vet Sci. 2013;16(2):293-7.
The aim of the study was to assess the impact of modulating factors on vascular smooth muscle cells reactivity. Vascular resistance was induced by the administration of increasing concentrations of imidazole. The experiments were performed on isolated and perfused tail artery of Wistar rats (weight 250 g - 350 g). Rats were been narcotized by urethane (intraperitoneal injection) at a dose of 120 mg/kg, stunned and then sacrificed by cervical dislocation. In the following investigation classical pharmacometric methods were used. Relationships between concentration-response curves (CRCs) for imidazole observed in the presence of ODQ [(1H-(1,2,4)oxadiazolo-[4,3-a]quinoxalin-1-one)], 7-nitroindazole and indomethacin were analyzed. Imidazole-induced contractility of vascular smooth muscle cells was independent from alpha-adrenergic receptors and PLC activity. Reactivity of VSMCsinduced by imidazole, was significantly changed in the presence of ODQ and 7-nitroindazole.
U-73122 reduces the cell growth in cultured MG-63 ostesarcoma cell line involving Phosphoinositide-specific Phospholipases C.[Pubmed:27026853]
Springerplus. 2016 Feb 24;5:156.
The definition of the number and nature of the signal transduction pathways involved in the pathogenesis and the identification of the molecules promoting metastasis spread might improve the knowledge of the natural history of osteosarcoma, also allowing refine the prognosis and opening the way to novel therapeutic strategies. Phosphatydil inositol (4,5) bisphosphate (PIP2), belonging to the Phosphoinositide (PI) signal transduction pathway, was related to the regulation of ezrin, an ezrin-radixin-moesin protein involved in metastatic osteosarcoma spread. The levels of PIP2 are regulated by means of the PI-specific Phospholipase C (PLC) enzymes. Recent literature data suggested that in osteosarcoma the panel of expression of PLC isoforms varies in a complex and unclear manner and is related to ezrin, probably networking with Ras GTPases, such as RhoA and Rac1. We analyzed the expression and the subcellular localization of PLC enzymes in cultured human osteosarcoma MG-63 cells, commonly used as an experimental model for human osteoblasts, using U-73122 PLC inhibitor, U-73343 inactive analogue, and by silencing ezrin. The treatment with U-73122 significantly reduces the number of MG-63 viable cells and contemporarily modifies the expression and the subcellular localization of selected PLC isoforms. U-73122 reduces the cell growth in cultured MG-63 ostesarcoma cell line involving PI-specific Phospholipases C.
The PI-PLC inhibitor U-73122 is a potent inhibitor of the SERCA pump in smooth muscle.[Pubmed:20590620]
Br J Pharmacol. 2010 Jul;160(6):1293-4.
In this issue MacMillan and McCarron in 2010 demonstrated that the phospholipase C (PLC) inhibitor U-73122 can potently inhibit Ca(2+) release from isolated smooth muscle cells independent of its effect on PLC. Their data suggest that the PLC inhibitor can block the sarcoplasmic/endoplasmic reticulum calcium ATPase pump in smooth muscle and cast doubt on the reliability of U-73122 as the main pharmacological tool to assess the role of the phosphotidyl inositol-PLC pathway in cellular signalling.
The phospholipase C inhibitor U-73122 inhibits Ca(2+) release from the intracellular sarcoplasmic reticulum Ca(2+) store by inhibiting Ca(2+) pumps in smooth muscle.[Pubmed:20590621]
Br J Pharmacol. 2010 Jul;160(6):1295-301.
BACKGROUND AND PURPOSE: The sarcoplasmic reticulum (SR) releases Ca(2+) via inositol 1,4,5-trisphosphate receptors (IP(3)R) in response to IP(3)-generating agonists. Ca(2+) release subsequently propagates as Ca(2+) waves. To clarify the role of IP(3) production in wave generation, the contribution of a key enzyme in the production of IP(3) was examined using a phosphoinositide-specific phospholipase C (PI-PLC) inhibitor, U-73122. EXPERIMENTAL APPROACH: Single colonic myocytes were voltage-clamped in whole-cell configuration and cytosolic Ca(2+) concentration ([Ca(2+)](cyto)) measured using fluo-3. SR Ca(2+) release was evoked either by activation of IP(3)Rs (by carbachol or photolysis of caged IP(3)) or ryanodine receptors (RyRs; by caffeine). KEY RESULTS: U-73122 inhibited carbachol-evoked [Ca(2+)](cyto) transients. The drug also inhibited [Ca(2+)](cyto) increases, evoked by direct IP(3)R activation (by photolysis of caged IP(3)) and RyR activation (by caffeine), which do not require PI-PLC activation. U-73122 also increased steady-state [Ca(2+)](cyto) and slowed the rate of Ca(2+) removal from the cytoplasm. An inactive analogue of U-73122, U-73343, was without effect on either IP(3)R- or RyR-mediated Ca(2+) release. CONCLUSIONS AND IMPLICATIONS: U-73122 inhibited carbachol-evoked [Ca(2+)](cyto) increases. However, the drug also reduced Ca(2+) release when evoked by direct activation of IP(3)R or RyR, slowed Ca(2+) removal and increased steady-state [Ca(2+)](cyto). These results suggest U-73122 reduces IP(3)-evoked Ca(2+) transients by inhibiting the SR Ca(2+) pump to deplete the SR of Ca(2+) rather than by inhibiting PI-PLC.
Direct modulation of TRPM4 and TRPM3 channels by the phospholipase C inhibitor U73122.[Pubmed:27328745]
Br J Pharmacol. 2016 Aug;173(16):2555-69.
BACKGROUND AND PURPOSE: Signalling through phospholipase C (PLC) controls many cellular processes. Much information on the relevance of this important pathway has been derived from pharmacological inhibition of the enzymatic activity of PLC. We found that the most frequently employed PLC inhibitor, U73122, activates endogenous ionic currents in widely used cell lines. Given the extensive use of U73122 in research, we set out to identify these U73122-sensitive ion channels. EXPERIMENTAL APPROACH: We performed detailed biophysical analysis of the U73122-induced currents in frequently used cell lines. KEY RESULTS: At concentrations required to inhibit PLC, U73122 modulated the activity of transient receptor potential melastatin (TRPM) channels through covalent modification. U73122 was shown to be a potent agonist of ubiquitously expressed TRPM4 channels and activated endogenous TRPM4 channels in CHO cells independently of PLC and of the downstream second messengers PI(4,5)P2 and Ca(2+) . U73122 also potentiated Ca(2) (+) -dependent TRPM4 currents in human Jurkat T-cells, endogenous TRPM4 in HEK293T cells and recombinant human TRPM4. In contrast to TRPM4, TRPM3 channels were inhibited whereas the closely related TRPM5 channels were insensitive to U73122, showing that U73122 exhibits high specificity within the TRPM channel family. CONCLUSIONS AND IMPLICATIONS: Given the widespread expression of TRPM4 and TRPM3 channels, these actions of U73122 must be considered when interpreting its effects on cell function. U73122 may also be useful for identifying and characterizing TRPM channels in native tissue, thus facilitating the analysis of their physiology.
Phospholipase C{beta}3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain.[Pubmed:19066214]
Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):20004-8.
Treatment of neuropathic pain is a major clinical problem. This study shows expression of phospholipase ss3 (PLCss3) in mouse and human DRG neurons, mainly in small ones and mostly with a nonpeptidergic phenotype. After spared nerve injury, the pain threshold was strongly reduced, and systemic treatment of such animals with the unselective PLC inhibitor U73122 caused a rapid and long-lasting (48-h) increase in pain threshold. Thus, inhibition of PLC may provide a way to treat neuropathic pain.
U-73122: a potent inhibitor of human polymorphonuclear neutrophil adhesion on biological surfaces and adhesion-related effector functions.[Pubmed:8764366]
J Pharmacol Exp Ther. 1996 Jul;278(1):320-9.
We have reported that U-73122 (1-[6-[[17 beta-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole- 2,5-dione) an inhibitor of phospholipase C-dependent processes in human polymorphonuclear neutrophils (PMN) and platelets, potently suppresses the responsiveness of suspended PMN and platelets to receptor agonists. We demonstrate here that U-73122 caused a concentration-dependent (10-800 nM) inhibition of N-formyl-methionyl-leucyl-phenylalanine, tumor necrosis factor-alpha (TNF alpha), interleukin-8 and phorbol myristate acetate (PMA)-triggered PMN adhesion on fibronectin, fetal bovine serum or keyhole limpet hemocyanincoated microtiter plates. U-73122 also inhibited PMN adherence to and transmigration through TNF-alpha-activated endothelium (IC50 < 50 nM). Further, U-73122 suppressed interleukin-8, N-formylmethionyl-leucyl-phenylalanine and PMA-stimulated up-regulation of the beta 2-integrin, Mac-1 (CD11b/CD18), on the PMN surface (IC50 < 1.3 microM). U-73122 also caused a time-(15-120 min) and concentration-dependent inhibition (IC50 = 25-100 nM) of the N-formyl-methionyl-leucyl-phenylalanine-, TNF alpha- and PMA-elicited adhesion-dependent, oxidative burst, measured as hydrogen peroxide (H2O2) production, in PMN. The CD18-dependent extracellular release of lactoferrin from PMN activated with these stimuli was also suppressed by U-73122. U-73343 (1-[6-[[17 beta-3- methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrrolidine dione), a close analog of U-73122, did not affect PMN responsiveness.


