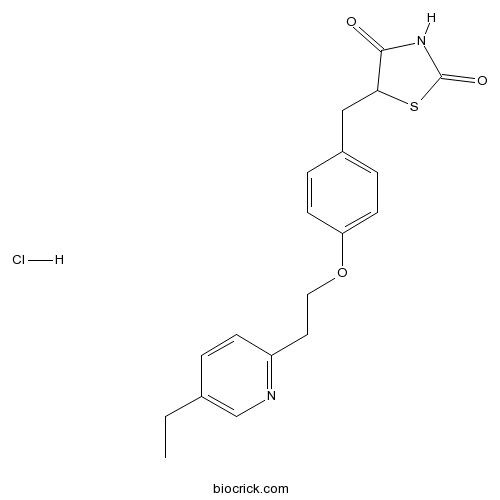
Pioglitazone HClPPARγ agonist CAS# 112529-15-4 |
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112529-15-4 | SDF | Download SDF |
| PubChem ID | 60560 | Appearance | Powder |
| Formula | C19H21ClN2O3S | M.Wt | 392.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | U 72107A; AD 4833 | ||
| Solubility | DMSO : 100 mg/mL (254.52 mM; Need ultrasonic) | ||
| Chemical Name | 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione;hydrochloride | ||
| SMILES | CCC1=CN=C(C=C1)CCOC2=CC=C(C=C2)CC3C(=O)NC(=O)S3.Cl | ||
| Standard InChIKey | GHUUBYQTCDQWRA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20N2O3S.ClH/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17;/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective PPARγ agonist (EC50 = 0.69 μM). Thiazolidinedione (TZD) derivative and antidiabetic agent; improves insulin sensitivity. |

Pioglitazone HCl Dilution Calculator

Pioglitazone HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5452 mL | 12.7259 mL | 25.4518 mL | 50.9035 mL | 63.6294 mL |
| 5 mM | 0.509 mL | 2.5452 mL | 5.0904 mL | 10.1807 mL | 12.7259 mL |
| 10 mM | 0.2545 mL | 1.2726 mL | 2.5452 mL | 5.0904 mL | 6.3629 mL |
| 50 mM | 0.0509 mL | 0.2545 mL | 0.509 mL | 1.0181 mL | 1.2726 mL |
| 100 mM | 0.0255 mL | 0.1273 mL | 0.2545 mL | 0.509 mL | 0.6363 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pioglitazone HCl is a potent and highly selective agonist of PPARγ with IC50 value of 3μM[1].
Pioglitazone HCl can increase both insulin-stimulated glucose uptake in peripheral tissues and insulin sensitivity in hepatic and adipose tissue. It is treated for hyperglycemia and Type 2 diabetes (T2D). Pioglitazone needs insulin as a co-factor for PPAR agonism so it is not effect when being treated to late stages of T2D with developed insulinopenia. In clinical trials, 15mg pioglitazone produces significant improvements in HbA1c and fasting plasma glucose (FPG) [2].
Pioglitazone is also reported to have multiple beneficial effects on lipid metabolism. It produces significant decreases in TG and significant increases in high density lipoprotein (HDL) in clinical studies [2].
References:
[1] Saad S, Agapiou DJ, Chen XM, Stevens V, Pollock CA. The role of Sgk-1 in the upregulation of transport proteins by PPAR-{gamma} agonists in human proximal tubule cells. Nephrol Dial Transplant. 2009 Apr;24(4):1130-41.
[2] Dorkhan M, Frid A. A review of pioglitazone HCL and glimepiride in the treatment of type 2 diabetes. Vasc Health Risk Manag. 2007;3(5):721-31.
- ent-16alpha,17-Dihydroxyatisan-3-one
Catalog No.:BCN6607
CAS No.:112523-91-8
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Citrusinol
Catalog No.:BCN8083
CAS No.:112516-43-5
- Tubuloside A
Catalog No.:BCN2806
CAS No.:112516-05-9
- Neolinine
Catalog No.:BCN6564
CAS No.:112515-37-4
- Isoabsouline
Catalog No.:BCN1955
CAS No.:112513-34-5
- Absouline
Catalog No.:BCN1954
CAS No.:112513-33-4
- Picrasidine S
Catalog No.:BCN6006
CAS No.:112503-87-4
- Aristolactam FI
Catalog No.:BCN6005
CAS No.:112501-42-5
- 6-Aldehydo-isoophiopogonone A
Catalog No.:BCN6629
CAS No.:112500-90-0
- 5-Aminoisoquinoline
Catalog No.:BCC8736
CAS No.:1125-60-6
- 4,4-Pentamethylenepiperidine hydrochloride
Catalog No.:BCC6059
CAS No.:1125-01-5
- 4-O-Methylepisappanol
Catalog No.:BCN3674
CAS No.:112529-37-0
- Mps1-IN-1
Catalog No.:BCC5590
CAS No.:1125593-20-5
- A 804598
Catalog No.:BCC6198
CAS No.:1125758-85-1
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- 4-Allylpyrocatechol
Catalog No.:BCN6009
CAS No.:1126-61-0
- Iso-mogroside V
Catalog No.:BCN3047
CAS No.:1126032-65-2
- Dicyclanil
Catalog No.:BCC8938
CAS No.:112636-83-6
- U-73122
Catalog No.:BCC5199
CAS No.:112648-68-7
- Garcinone E
Catalog No.:BCN3604
CAS No.:112649-21-5
- BR-Xanthone A
Catalog No.:BCN6007
CAS No.:112649-48-6
- 1,7-Dihydroxyacridone
Catalog No.:BCN7275
CAS No.:112649-95-3
- Fragransin A2
Catalog No.:BCN6008
CAS No.:112652-46-7
A Pilot Trial of Pioglitazone HCl and Tretinoin in ALS: Cerebrospinal Fluid Biomarkers to Monitor Drug Efficacy and Predict Rate of Disease Progression.[Pubmed:22830016]
Neurol Res Int. 2012;2012:582075.
Objectives. To determine if therapy with Pioglitazone HCl and tretinoin could slow disease progression in patients with ALS. Levels of tau and pNFH in the cerebrospinal fluid were measured to see if they could serve as prognostic indicators. Methods. 27 subjects on stable doses of riluzole were enrolled. Subjects were randomized to receive pioglitazone 30 mg/d and tretinoin 10 mg/BID for six months or two matching placebos. ALSFRS-R scores were followed monthly. At baseline and at the final visit, lumbar punctures (LPs) were performed to measure cerebrospinal fluid (CSF) biomarker levels. Results. Subjects treated with tretinoin, pioglitazone, and riluzole had an average rate of decline on the ALSFRS-R scale of -1.02 points per month; subjects treated with placebo and riluzole had a rate of decline of -.86 (P = .18). Over six months of therapy, CSF tau levels decreased in subjects randomized to active treatment and increased in subjects on placebo. Further higher levels of pNF-H at baseline correlated with a faster rate of progression. Conclusion. ALS patients who were treated with tretinoin and pioglitazone demonstrated no slowing on their disease progression. Interestingly, the rate of disease progression was strongly correlated with levels of pNFH in the CSF at baseline.
A review of pioglitazone HCL and glimepiride in the treatment of type 2 diabetes.[Pubmed:18078023]
Vasc Health Risk Manag. 2007;3(5):721-31.
Type 2 diabetes (T2D) is a progressive disorder with a consistent and steady increase in glycosylated hemoglobin (HbA1c) over time associated with enhanced risk of micro- and macrovascular complications and a substantial reduction in life expectancy. There are three major pathophysiologic abnormalities associated with T2D: impaired insulin secretion, excessive hepatic glucose output, and insulin resistance in skeletal muscle, liver, and adipose tissue. These defects have been treated in clinical praxis by use of oral insulin secretagogues (sulfonylureas/ glinides) or insulin, biguanides, and thiazolidinediones (TZDs) respectively. Pioglitazone HCl is an insulin sensitizer in the TZD family and glimepiride is an insulin secretagogue in the SU family. This article reviews mechanisms of action and clinical data behind the use of these two commonly used oral hypoglycemic agents with documented efficacy and good safety profile of once-daily administration, alone or in combination with insulin or metformin, in the management of T2D in terms of glycemic and non-glycemic effects, tolerability and side effects, and impact on vascular health.
Flow-injection chemiluminometric determination of pioglitazone HCl by its sensitizing effect on the cerium-sulfite reaction.[Pubmed:19276598]
Anal Sci. 2009 Mar;25(3):401-6.
A flow injection chemiluminescent (FI-CL) method was developed for the determination of Pioglitazone HCl. It is based on the sensitizing effect of the drug on the oxidation reaction of sulfite with cerium(IV). The different experimental parameters affecting the chemiluminescence intensity, such as concentration of reagents and some physical parameters of the manifold, were carefully studied and incorporated into the procedure. The method permits the determination of 0.05 - 3.0 microg ml(-1) of Pioglitazone HCl with correlation coefficient r = 0.9999. The lower limit of detection (LOD) is 0.01 microg ml(-1) (S/N = 2) and the lower limit of quantitation (LOQ) is 0.05 microg ml(-1). The proposed method was compared with other reported methods and was found to be equally accurate and precise. It was successfully applied to the determination of the drug in pharmaceutical preparations and in biological fluids.
A Validated Adsorptive Stripping Voltammetric Determination of Antidiabetic Agent Pioglitazone HCl in Tablets and Biological Fluids.[Pubmed:23675103]
Int J Biomed Sci. 2008 Dec;4(4):310-8.
Square-wave adsorptive cathodic stripping voltammetry was used to determine Pioglitazone HCl in Britton Robinson buffer of pH5. The adsorptive cathodic peak was observed at -1.5 V vs. Ag/AgCl. The peak response was characterized with respect to pH, supporting electrolyte, frequency, scan increment, pulse-amplitude, accumulation potential and pre-concentration time. Under optimal conditions, the peak current is proportional to the concentration of Pioglitazone HCl, and a linear calibration graphs were obtained within the concentration levels of 10(-8) and 10(-4) M following different accumulation time periods (0-300 s). The obtained results were analyzed and the statistical parameters were calculated. The detection limit is 8.08 x 10(-9) M (3.17 ng ml(-1)) using 300 s pre-concentration time, whereas the quantitative limit is 2.45 x 10(-8) M (9.63 ng ml(-1)). The proposed method was applied to assay the drug in pharmaceutical formulations and biological fluids. The pharmacokinetic parameters of drug in human plasma were estimated as: C max=785.8 ng ml(-1), t max=1.5 h, K e=0.125 h(-1) and t 1/2=8 h which are favorably compared with those reported in literature.
Novel 5-substituted 2,4-thiazolidinedione and 2,4-oxazolidinedione derivatives as insulin sensitizers with antidiabetic activities.[Pubmed:11906293]
J Med Chem. 2002 Mar 28;45(7):1518-34.
Two novel classes of 2,4-thiazolidinediones and 2,4-oxazolidinediones with an omega-(azolylalkoxyphenyl)alkyl substituent at the 5-position were prepared and their antidiabetic effects were evaluated in two genetically obese and diabetic animal models, KKA(y) mice and Wistar fatty rats. A large number of the 2,4-thia(oxa)zolidinediones showed potent glucose- and lipid-lowering activities. The antidiabetic activities of the 2,4-oxazolidinediones were superior to those of the 2,4-thiazolidinediones. Among the compounds, both enantiomers of 5-[3-[4-[2-(2-furyl)-5-methyl-4-oxazolylmethoxy]-3-methoxyphenyl]propyl]-2,4-oxaz olidinedione (64), one of the most interesting compounds in terms of activity, were synthesized by using an asymmetric O-acetylation of the corresponding alpha-hydroxyvalerate (26) with immobilized lipase, followed by cyclization of the oxazolidinedione ring. (R)-(+)-64 showed more potent glucose-lowering activity (effective dose (ED)25 = 0.561 mg/kg/d) than (S)-(-)-64 (ED25 > 1.5 mg/kg/d) or pioglitazone (ED25 = 6 mg/kg/d) in KKA(y) mice. It also exhibited a 10-fold more potent antidiabetic activity (ED25 = 0.05 mg/kg/d) than pioglitazone (ED25 = 0.5 mg/kg/d) in Wistar fatty rats. The antidiabetic effects of this compound are considered to be due to its potent agonistic activity for peroxisome proliferator-activated receptor gamma (EC(50) = 8.87 nM).
Pioglitazone: mechanism of action.[Pubmed:11594239]
Int J Clin Pract Suppl. 2001 Sep;(121):13-8.
Thiazolidinediones, such as pioglitazone, are synthetic ligands for peroxisome proliferator-activated receptors (PPARs). They alter the transcription of genes influencing carbohydrate and lipid metabolism, resulting in changed amounts of protein synthesis and, therefore, metabolic changes. Pioglitazone improves glycaemic control in people with Type 2 diabetes by improving insulin sensitivity through its action at PPAR gamma 1 and PPAR gamma 2, and affects lipid metabolism through action at PPAR alpha. The results of these interactions include increases in glucose transporters 1 and 4, lowered free fatty acids, enhanced insulin signalling, reduced tumour necrosis factor alpha (TNF alpha) and remodelling of adipose tissue. Together, these can increase glucose uptake and utilisation in the peripheral organs and decrease gluconeogenesis in the liver, thereby reducing insulin resistance.
Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone.[Pubmed:11095972]
Biochem Biophys Res Commun. 2000 Nov 30;278(3):704-11.
Pioglitazone, a thiazolidinedione (TZD) derivative, is an antidiabetic agent that improves hyperglycaemia and hyperlipidaemia in obese and diabetic animals via a reduction in hepatic and peripheral insulin resistance. The TZDs including pioglitazone have been identified as high affinity ligands for peroxisome proliferator-activated receptor (PPAR) gamma. The selectivity of pioglitazone for the human PPAR subtypes has not been reported, thus, we investigated the effect of pioglitazone on the human PPAR subtypes. Transient transactivation assay showed that pioglitazone is a selective hPPARgamma1 activator and a weak hPPARalpha activator. Binding assay indicated that the transactivation of hPPARgamma1 or hPPARalpha by pioglitazone is due to direct binding of pioglitazone to each subtype. Furthermore, pioglitazone significantly increased the apoA-I secretion from the human hepatoma cell line HepG2.


