AbsoulineCAS# 112513-33-4 |
- Isoabsouline
Catalog No.:BCN1955
CAS No.:112513-34-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112513-33-4 | SDF | Download SDF |
| PubChem ID | 6442818 | Appearance | Powder |
| Formula | C17H22N2O2 | M.Wt | 286.37 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
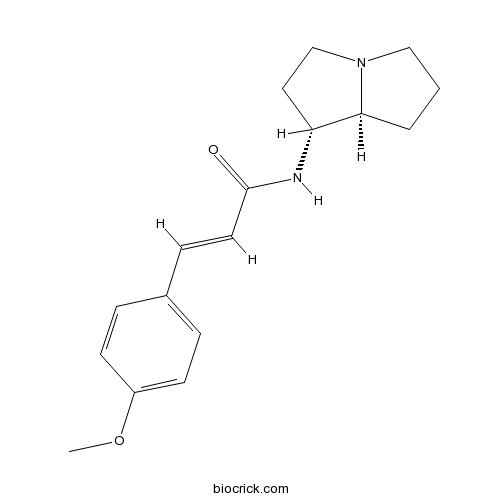
| Chemical Name | (E)-N-[(1R,8S)-2,3,5,6,7,8-hexahydro-1H-pyrrolizin-1-yl]-3-(4-methoxyphenyl)prop-2-enamide | ||
| SMILES | COC1=CC=C(C=C1)C=CC(=O)NC2CCN3C2CCC3 | ||
| Standard InChIKey | SBFIICJNXKHXND-GRLYAWNKSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Absouline is a natural product from Hugonia oreogena. |
| Structure Identification | Tetrahedron, 2000 , 56 (13) :1837-1850Synthèse des 1-amidopyrrolizidines naturelles, absouline et laburnamine, de dérivés et d'analogues pyrrolidinoimidazoliques[Reference: WebLink]Natural 1-amidopyrrolizidines, Absouline and laburnamine, were synthesized via stable pyrrolizidin-1-one hydrobromide. Amides, ester derivatives and aminopyrrolidinoimidazole analogues were also prepared and their biological activities tested. |

Absouline Dilution Calculator

Absouline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.492 mL | 17.4599 mL | 34.9199 mL | 69.8397 mL | 87.2996 mL |
| 5 mM | 0.6984 mL | 3.492 mL | 6.984 mL | 13.9679 mL | 17.4599 mL |
| 10 mM | 0.3492 mL | 1.746 mL | 3.492 mL | 6.984 mL | 8.73 mL |
| 50 mM | 0.0698 mL | 0.3492 mL | 0.6984 mL | 1.3968 mL | 1.746 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3492 mL | 0.6984 mL | 0.873 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Picrasidine S
Catalog No.:BCN6006
CAS No.:112503-87-4
- Aristolactam FI
Catalog No.:BCN6005
CAS No.:112501-42-5
- 6-Aldehydo-isoophiopogonone A
Catalog No.:BCN6629
CAS No.:112500-90-0
- 5-Aminoisoquinoline
Catalog No.:BCC8736
CAS No.:1125-60-6
- 4,4-Pentamethylenepiperidine hydrochloride
Catalog No.:BCC6059
CAS No.:1125-01-5
- H-Glu(OcHex)-OH
Catalog No.:BCC2929
CAS No.:112471-82-6
- U-54494A hydrochloride
Catalog No.:BCC6668
CAS No.:112465-94-8
- 3-Phenyl-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN4005
CAS No.:112448-69-8
- AZ3146
Catalog No.:BCC3731
CAS No.:1124329-14-1
- Ganoderic acid Jb
Catalog No.:BCN7972
CAS No.:112430-68-9
- Ganoderic acid T-Q
Catalog No.:BCN3209
CAS No.:112430-66-7
- Ganoderic acid TN
Catalog No.:BCN2443
CAS No.:112430-64-5
- Isoabsouline
Catalog No.:BCN1955
CAS No.:112513-34-5
- Neolinine
Catalog No.:BCN6564
CAS No.:112515-37-4
- Tubuloside A
Catalog No.:BCN2806
CAS No.:112516-05-9
- Citrusinol
Catalog No.:BCN8083
CAS No.:112516-43-5
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- ent-16alpha,17-Dihydroxyatisan-3-one
Catalog No.:BCN6607
CAS No.:112523-91-8
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- 4-O-Methylepisappanol
Catalog No.:BCN3674
CAS No.:112529-37-0
- Mps1-IN-1
Catalog No.:BCC5590
CAS No.:1125593-20-5
- A 804598
Catalog No.:BCC6198
CAS No.:1125758-85-1
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- 4-Allylpyrocatechol
Catalog No.:BCN6009
CAS No.:1126-61-0
Synthesis of diamino carboxylic esters by palladium-catalyzed oxidative intramolecular diamination of acrylates.[Pubmed:18655067]
Chem Asian J. 2008 Sep 1;3(8-9):1248-55.
Unligated palladium(II) salts catalyze the oxidative diamination of acrylic esters to yield 2,3-diamino carboxylic esters. The reaction employs copper(II) bromide as oxidant and proceeds with good to excellent stereoselectivities and complete chemoselectivity. Preliminary mechanistic studies provide evidence for the involvement of a direct amination of the C--Pd bond in the alpha position relative to the ester group. This protocol significantly broadens the overall scope of the palladium-catalyzed diamination of alkenes and represents the first direct diamination of functionalized nonterminal substrates. The reaction yields readily protected 2,3-diamino acid derivatives, which can be considered as highly functionalized building blocks for subsequent synthesis. The use of one of these new diamination products as a suitable starting material in a short synthesis of the alkaloid Absouline is demonstrated as an example.
3-Aminopyrrolidines via ring rearrangement of 2-aminomethylazetidines. Synthesis of (-)-absouline.[Pubmed:16354085]
Org Lett. 2005 Dec 22;7(26):5861-4.
[reaction: see text] A new entry to enantiopure 3-aminopyrrolidines was developed using a boron trifluoride-mediated rearrangement of 2-aminomethylazetidines. The method is quite general and produces rearranged products in good yield regardless of both substitution pattern and relative stereochemistry of the starting material. A concise stereocontrolled synthesis of (-)-Absouline was achieved on the basis of this new method.


