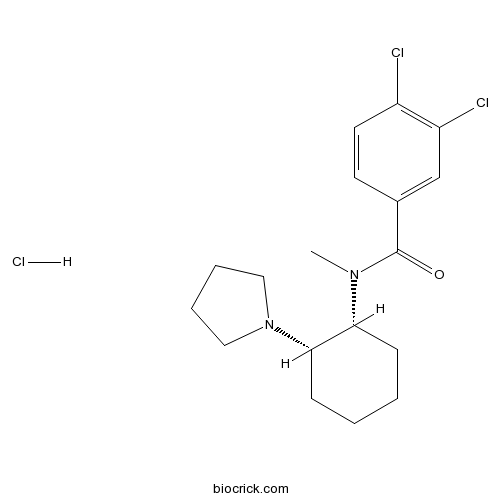
U-54494A hydrochlorideCAS# 112465-94-8 |
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Dorzolamide HCl
Catalog No.:BCC2311
CAS No.:130693-82-2
- Brinzolamide
Catalog No.:BCC2313
CAS No.:138890-62-7
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112465-94-8 | SDF | Download SDF |
| PubChem ID | 183469 | Appearance | Powder |
| Formula | C18H25Cl3N2O | M.Wt | 391.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 3,4-dichloro-N-methyl-N-[(1R,2S)-2-pyrrolidin-1-ylcyclohexyl]benzamide;hydrochloride | ||
| SMILES | CN(C1CCCCC1N2CCCC2)C(=O)C3=CC(=C(C=C3)Cl)Cl.Cl | ||
| Standard InChIKey | WFUASZXAHZXJMX-PPPUBMIESA-N | ||
| Standard InChI | InChI=1S/C18H24Cl2N2O.ClH/c1-21(18(23)13-8-9-14(19)15(20)12-13)16-6-2-3-7-17(16)22-10-4-5-11-22;/h8-9,12,16-17H,2-7,10-11H2,1H3;1H/t16-,17+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | κ-opioid agonist and anticonvulsant. Perhaps acts as an NMDA antagonist. |

U-54494A hydrochloride Dilution Calculator

U-54494A hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5525 mL | 12.7626 mL | 25.5252 mL | 51.0504 mL | 63.813 mL |
| 5 mM | 0.5105 mL | 2.5525 mL | 5.105 mL | 10.2101 mL | 12.7626 mL |
| 10 mM | 0.2553 mL | 1.2763 mL | 2.5525 mL | 5.105 mL | 6.3813 mL |
| 50 mM | 0.0511 mL | 0.2553 mL | 0.5105 mL | 1.021 mL | 1.2763 mL |
| 100 mM | 0.0255 mL | 0.1276 mL | 0.2553 mL | 0.5105 mL | 0.6381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Phenyl-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN4005
CAS No.:112448-69-8
- AZ3146
Catalog No.:BCC3731
CAS No.:1124329-14-1
- Ganoderic acid Jb
Catalog No.:BCN7972
CAS No.:112430-68-9
- Ganoderic acid T-Q
Catalog No.:BCN3209
CAS No.:112430-66-7
- Ganoderic acid TN
Catalog No.:BCN2443
CAS No.:112430-64-5
- 2',5,7-Trihydroxy-8-methoxyflavanone
Catalog No.:BCN6004
CAS No.:112408-71-6
- 3'-Deoxy-4-O-methylsappanol
Catalog No.:BCN3675
CAS No.:112408-68-1
- 3-Deoxysappanchalcone
Catalog No.:BCN3736
CAS No.:112408-67-0
- Tetramethylpyrazine
Catalog No.:BCN1008
CAS No.:1124-11-4
- XL765
Catalog No.:BCC2060
CAS No.:1123889-87-1
- DMPQ dihydrochloride
Catalog No.:BCC6977
CAS No.:1123491-15-5
- WAY-262611
Catalog No.:BCC5507
CAS No.:1123231-07-1
- H-Glu(OcHex)-OH
Catalog No.:BCC2929
CAS No.:112471-82-6
- 4,4-Pentamethylenepiperidine hydrochloride
Catalog No.:BCC6059
CAS No.:1125-01-5
- 5-Aminoisoquinoline
Catalog No.:BCC8736
CAS No.:1125-60-6
- 6-Aldehydo-isoophiopogonone A
Catalog No.:BCN6629
CAS No.:112500-90-0
- Aristolactam FI
Catalog No.:BCN6005
CAS No.:112501-42-5
- Picrasidine S
Catalog No.:BCN6006
CAS No.:112503-87-4
- Absouline
Catalog No.:BCN1954
CAS No.:112513-33-4
- Isoabsouline
Catalog No.:BCN1955
CAS No.:112513-34-5
- Neolinine
Catalog No.:BCN6564
CAS No.:112515-37-4
- Tubuloside A
Catalog No.:BCN2806
CAS No.:112516-05-9
- Citrusinol
Catalog No.:BCN8083
CAS No.:112516-43-5
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
Modulation of release of acetylcholine from the striatum by a proposed excitatory amino acid antagonist U-54494A: comparison with known antagonists, diazepam and phenytoin.[Pubmed:1348110]
Neuropharmacology. 1992 Feb;31(2):111-4.
The effect of (U-54494A) cis-3,4-dichloro-N-methyl-N-[2-(1-Pyrrolidinyl)- cyclohexyl] benzamide monohydrochloride, an excitatory amino acid antagonist, on N-methyl-D-aspartic acid (NMDA)- and K(+)-evoked release of [3H]acetylcholine [( 3H]ACh) from slices of striatum was investigated. For the purpose of comparison, MK 801, PCP, CGP 37849, CPP, phenytoin and diazepam were investigated under identical conditions. Both U-54494A and the excitatory amino acid antagonists blocked NMDA-evoked release of [3H]ACh but these compounds failed to inhibit K(+)-evoked release of this neurotransmitter. Phenytoin blocked both NMDA and K(+)-evoked release of [3H]ACh, whereas diazepam was ineffective under similar conditions. These observations indicate that excitatory amino acid antagonists, including U-54494A, may mediate their anticonvulsant effect by blocking the activity of NMDA receptors, diazepam by activating the benzodiazepine receptors and phenytoin by inhibiting the activity of various depolarizing agents.
In vitro depressant effects of U-54494A, an anticonvulsant related to kappa opioids, in the hippocampus.[Pubmed:1656303]
Neuropharmacology. 1991 Jun;30(6):637-42.
The effects of cis-3,4 dichloro-N-2-(1-pyrrolidinyl)cyclo-hexyl-benzamide (U-54494A), an anticonvulsant related to kappa opioids, were studied in vitro on the extracellular electrical activity of the CA1 region of slices of hippocampus in the rat. The effects of U-54494A were compared to those of the kappa opioid agonist trans-3,4 dichloro-N-2-(1-pyrrolidinyl)cyclo-hexyl benzeneacetamide methane sulphonate (U-50488H). Both U-54494A and U-50488H, in concentrations of 50 and 100 microM, respectively, reduced the magnitude of the orthodromically evoked CA1 population spikes after electrical stimulation of the stratum radiatum (100-200 microA, 70 microseconds, 0.1 Hz). Naltrexone (25 microM), or the selective kappa opiate receptor antagonist, 1-cyclopenthyl-5-(1,2,3,4,5,6-hexahydroxy-3,6,11-trimethyl-2 -6-methano-3- benzazocin)-3-pentatone methane sulphonate (WIN 44441-3) (25 microM), prevented the depressant activity of U-54494A (200 microM) on the CA1 population spikes. High calcium (+3mM) solutions prevented the depressant activity of increasing concentrations of both U-54494A and U-50488H on the amplitude of CA1 population spikes. Up to 200 microM, both drugs were ineffective in depressing the epileptiform bursting in CA1, due to 1 mM penicillin or to perfusion of the slice in absence of magnesium ions. The results demonstrate: (1) the inability of U-54494A to show antagonistic activity in two in vitro models of interictal epilepsy; (2) a depressant effect of U-54494A on basal synaptic transmission in the CA1 region of the hippocampus, which may be related to an influence on transneuronal calcium currents and which may be involved in the reported antagonism of ictal epileptic seizures by drugs.
U-54494A: a unique anticonvulsant related to kappa opioid agonists.[Pubmed:2824750]
J Pharmacol Exp Ther. 1987 Nov;243(2):542-7.
The benzamide U-54494A was compared to U-50488H (a structurally related kappa opioid agonist), phenytoin and phenobarbital in a variety of tests of anticonvulsant and sedative activities. In electroshock convulsion antagonism studies in mice and rats, U-54494A was generally similar to the standards in regard to milligrams of potency, threshold elevation, p.o. activity and duration of action. In contrast to phenytoin and phenobarbital, both U-54494A and U-50488H were effective antagonists of the convulsions induced by the excitatory amino acid agonists (kainic, N-methyl-aspartic and quisqualic acids) and the Ca++ channel agonist (Bay K 8644). They were not, however, effective antagonists of the gamma-aminobutyric acid-related convulsants (bicuculline and pentylenetetrazole), but all four blocked audiogenic convulsions in genetically epileptic mice. U-54494A in contrast to U-50488H lacks the kappa receptor-mediated sedative and analgesic activities but the anticonvulsant properties of both compounds are antagonized by high doses of naltrexone. Further investigations of the mechanism of action of these compounds revealed that both caused a dose-related suppression of post-tetanic repetitive discharge in cats soleus nerve-muscle preparations as measured by an abolition of the obligatory potentiation of soleus muscle contractile tension. On a biochemical level, both U-54494A and U-50488H attenuate the depolarization induced uptake of 45Ca++ into forebrain synaptosomes and block the enhancement of [3H]kainic acid binding induced by CaCl2. Together these results suggest that U-54494A is a unique and selective anticonvulsant agent acting by a Ca++-related mechanism possibly through a subclass of kappa receptors.


