TetramethylpyrazineCAS# 1124-11-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1124-11-4 | SDF | Download SDF |
| PubChem ID | 14296 | Appearance | White powder |
| Formula | C8H12N2 | M.Wt | 136.20 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Chuanxingzine; Tetramethylpyrazine; Tetrapyrazine | ||
| Solubility | DMSO : 50 mg/mL (367.13 mM; Need ultrasonic) | ||
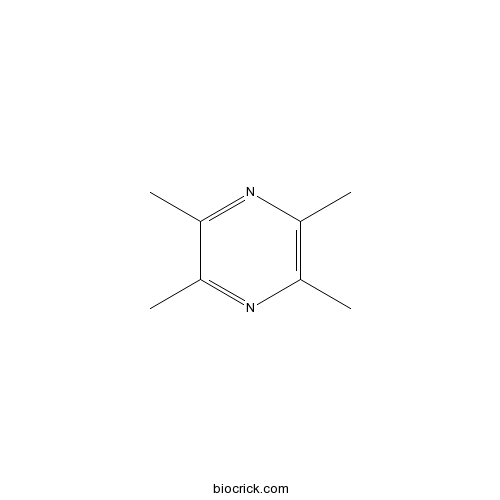
| Chemical Name | 2,3,5,6-tetramethylpyrazine | ||
| SMILES | CC1=C(N=C(C(=N1)C)C)C | ||
| Standard InChIKey | FINHMKGKINIASC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H12N2/c1-5-6(2)10-8(4)7(3)9-5/h1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tetramethylpyrazine has antiinflammatory, analgesic, antioxidant, antiplatelet, antitumor, hepatoprotective, and antiapoptosis activities. Tetramethylpyrazine exerts neuroprotective effect against hypoxia, it inhibits CoCl2 -induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1α/NOX2/ROS pathways. It targeted HSCs via PDGF-βR/NLRP3/caspase1 pathway. |
| Targets | Nrf2 | HIF | ROS | NO | PDGFR | Caspase | Calcium Channel | NADPH-oxidase | TNF-α | NF-kB | PARP | NOS | NLRP3 |
| In vitro | Tetramethylpyrazine inhibits CoCl2 -induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1α/NOX2/ROS pathways.[Pubmed: 25952107]J Neurochem. 2015 May 8.Hypoxia-mediated neurotoxicity contributes to various neurodegenerative disorders, including Alzheimer's disease and multiple sclerosis. Tetramethylpyrazine (TMP), a major bioactive component purified from Ligusticum wallichii Franchat, exhibited potent neuroprotective effect. However, the mechanism of TMP-exerted neuroprotective effect against hypoxia was not clear.
Tetramethylpyrazine reduces inflammation in liver fibrosis and inhibits inflammatory cytokine expression in hepatic stellate cells by modulating NLRP3 inflammasome pathway.[Pubmed: 25847612]IUBMB Life. 2015 Apr 3.Hepatic fibrosis is concomitant with liver inflammation, which has been highlighted as significant treatment of chronic liver disease.
We previously demonstrated that Tetramethylpyrazine (TMP), the effective component of Ligusticum chuanxiong Hort, can inhibit the activation of HSCs and consequential anti-hepatic fibrosis.
The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase.[Pubmed: 10946853]Life Sci., 2000, 67(8):937-47.Tetramethylpyrazine (TMPZ) is an active ingredient of a Chinese herbal medicine (Ligusticum wallichii Franchat).
|
| In vivo | Tetramethylpyrazine (TMP) exerts antitumor effects by inducing apoptosis and autophagy in hepatocellular carcinoma.[Pubmed: 25841319]Int Immunopharmacol. 2015 May;26(1):212-20.Hepatocellular carcinoma (HCC) is one of the most common types of liver cancers with high recurrence rate and mortality rate. Recent studies have indicated that Tetramethylpyrazine (TMP), a purified chemical extracted from Ligusticum wallichii Franchat (ChuanXiong), possessed antitumor effects on HCC, but detailed mechanism remains unclear.
Antiinflammatory effect of tetramethylpyrazine and ferulic acid.[Pubmed: 1525949]Chem Pharm Bull (Tokyo). 1992 Apr;40(4):954-6.Tetramethylpyrazine (TMP) is one of the alkaloids contained in Ligusticum wallichii Franch (L. wallichii). Ferulic acid (FA) is a phenolic compound contained in L. wallichii and Angelica sinensis (Oliv.) Diels (A. sinensis).
|
| Cell Research | Beneficial effects of tetramethylpyrazine, an active constituent of Chinese herbs, on rats with endotoxemia.[Pubmed: 9536520]Relaxation effect of a novel Danshensu/tetramethylpyrazine derivative on rat mesenteric arteries.[Pubmed: 25952729]Eur J Pharmacol. 2015 May 4. pii: S0014-2999(15)00403-3.Danshen (Radix Salviae miltiorrhizae) and ChuanXiong (Ligusticum wallichii) are two traditional herbal medicines commonly used in China for the treatment of cardiovascular diseases. The active components in Danshen and ChuanXiong are Danshensu (DSS, (R)-3, 4-dihydroxyphenyllactic acid) and Tetramethylpyrazine (TMP), respectively. In the present study, a new compound named ADTM, which is a conjugation of DSS and TMP, was synthesized and its effect on the contractility of rat mesenteric arteries was examined.
Proc Natl Sci Counc Repub China B. 1998 Jan;22(1):46-54.Cell lines:HL-60 cells |
| Animal Research | Neuroprotective effects of tetramethylpyrazine against dopaminergic neuron injury in a rat model of Parkinson's disease induced by MPTP.[Pubmed: 24719552 ]Int J Biol Sci. 2014 Mar 13;10(4):350-7.Animal Models: A rat model of Parkinson's disease induced by MPTP (Wistar rats) |

Tetramethylpyrazine Dilution Calculator

Tetramethylpyrazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3421 mL | 36.7107 mL | 73.4214 mL | 146.8429 mL | 183.5536 mL |
| 5 mM | 1.4684 mL | 7.3421 mL | 14.6843 mL | 29.3686 mL | 36.7107 mL |
| 10 mM | 0.7342 mL | 3.6711 mL | 7.3421 mL | 14.6843 mL | 18.3554 mL |
| 50 mM | 0.1468 mL | 0.7342 mL | 1.4684 mL | 2.9369 mL | 3.6711 mL |
| 100 mM | 0.0734 mL | 0.3671 mL | 0.7342 mL | 1.4684 mL | 1.8355 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- XL765
Catalog No.:BCC2060
CAS No.:1123889-87-1
- DMPQ dihydrochloride
Catalog No.:BCC6977
CAS No.:1123491-15-5
- WAY-262611
Catalog No.:BCC5507
CAS No.:1123231-07-1
- BRL 52537 hydrochloride
Catalog No.:BCC6751
CAS No.:112282-24-3
- H-Asp(OcHex)-OH
Catalog No.:BCC2887
CAS No.:112259-66-2
- 20(R)-Ginsenoside Rh2
Catalog No.:BCN2484
CAS No.:112246-15-8
- (S)-tert-Leucinol
Catalog No.:BCN8367
CAS No.:112245-13-3
- Stigmastane-3,6-diol
Catalog No.:BCN6003
CAS No.:112244-29-8
- 16-O-Methyl-14,15-didehydroisovincanol
Catalog No.:BCN1618
CAS No.:112237-71-5
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- DMAP
Catalog No.:BCC2842
CAS No.:1122-58-3
- 3-Deoxysappanchalcone
Catalog No.:BCN3736
CAS No.:112408-67-0
- 3'-Deoxy-4-O-methylsappanol
Catalog No.:BCN3675
CAS No.:112408-68-1
- 2',5,7-Trihydroxy-8-methoxyflavanone
Catalog No.:BCN6004
CAS No.:112408-71-6
- Ganoderic acid TN
Catalog No.:BCN2443
CAS No.:112430-64-5
- Ganoderic acid T-Q
Catalog No.:BCN3209
CAS No.:112430-66-7
- Ganoderic acid Jb
Catalog No.:BCN7972
CAS No.:112430-68-9
- AZ3146
Catalog No.:BCC3731
CAS No.:1124329-14-1
- 3-Phenyl-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN4005
CAS No.:112448-69-8
- U-54494A hydrochloride
Catalog No.:BCC6668
CAS No.:112465-94-8
- H-Glu(OcHex)-OH
Catalog No.:BCC2929
CAS No.:112471-82-6
- 4,4-Pentamethylenepiperidine hydrochloride
Catalog No.:BCC6059
CAS No.:1125-01-5
- 5-Aminoisoquinoline
Catalog No.:BCC8736
CAS No.:1125-60-6
Relaxation effect of a novel Danshensu/tetramethylpyrazine derivative on rat mesenteric arteries.[Pubmed:25952729]
Eur J Pharmacol. 2015 Aug 15;761:153-60.
Danshen (Radix Salviae miltiorrhizae) and ChuanXiong (Ligusticum wallichii) are two traditional herbal medicines commonly used in China for the treatment of cardiovascular diseases. The active components in Danshen and ChuanXiong are Danshensu (DSS, (R)-3, 4-dihydroxyphenyllactic acid) and Tetramethylpyrazine (TMP), respectively. In the present study, a new compound named ADTM, which is a conjugation of DSS and TMP, was synthesized and its effect on the contractility of rat mesenteric arteries was examined. The relaxation effect of ADTM on rat mesenteric arteries was studied using myography. The effects of ADTM on Ca(2+) channels were measured by Ca(2+) imaging and patch-clamp techniques. The results showed that ADTM caused a concentration-dependent relaxation of rat mesenteric arteries. This relaxation effect was not affected by the removal of endothelium or inhibitors of nitric oxide synthase, cyclooxygenase, guanylyl cyclase and adenylyl cyclase. Potassium channel blockers including tetraethylammonium, iberiotoxin, apamin, 4-aminopyridine, BaCl2 and glibenclamide also failed to inhibit the relaxation response to ADTM. ADTM inhibited CaCl2-induced contractions and reduced the Ca(2+) influx in isolated mesenteric arterial muscle cells. Our results suggest that ADTM may be a novel relaxing agent. Its mechanism of action involves the direct blockade of voltage-gated Ca(2+) channels in vascular smooth muscle cells, resulting in a decrease in Ca(2+) influx into the cells.
Antiinflammatory effect of tetramethylpyrazine and ferulic acid.[Pubmed:1525949]
Chem Pharm Bull (Tokyo). 1992 Apr;40(4):954-6.
Tetramethylpyrazine (TMP) is one of the alkaloids contained in Ligusticum wallichii Franch (L. wallichii). Ferulic acid (FA) is a phenolic compound contained in L. wallichii and Angelica sinensis (Oliv.) Diels (A. sinensis). The present study was carried out to examine the antiinflammatory effect and to elucidate the mode of the effect of TMP and FA. Both compounds significantly inhibited the edema induced by carrageenin, the increase of the dye leakage induced by acetic acid and the granuloma formation induced by cotton pellet. And also, TMP and FA inhibited the number of writhes induced by acetic acid. From these results, it is suggested that both compounds have the antiinflammatory effect and the analgesic effect, and both compounds exert an antiinflammatory effect at the early and the late stages of processes in the inflammatory pathology.
The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase.[Pubmed:10946853]
Life Sci. 2000 Jul 14;67(8):937-47.
Tetramethylpyrazine (TMPZ) is an active ingredient of a Chinese herbal medicine (Ligusticum wallichii Franchat). In this study, TMPZ (50-200 microM) significantly increased production of nitrate and cyclic GMP in human platelets within a 15-min incubation period. TMPZ concentration-dependently inhibited intracellular Ca2+ mobilization in human platelets stimulated by collagen (5 microg/ml). Furthermore, TMPZ concentration (50 and 200 microM)- and time (15 and 30 min)-dependently triggered endothelial-type constitutive nitric oxide synthase (ecNOS) protein expression in human platelets. These results indicated that TMPZ at micromolar concentrations stimulated nitric oxide production in human platelets via a novel mechanism that activated ecNOS protein expression.
Tetramethylpyrazine reduces inflammation in liver fibrosis and inhibits inflammatory cytokine expression in hepatic stellate cells by modulating NLRP3 inflammasome pathway.[Pubmed:25847612]
IUBMB Life. 2015 Apr;67(4):312-21.
Hepatic fibrosis is concomitant with liver inflammation, which has been highlighted as significant treatment of chronic liver disease. We previously demonstrated that Tetramethylpyrazine (TMP), the effective component of Ligusticum chuanxiong Hort, can inhibit the activation of HSCs and consequential anti-hepatic fibrosis. In this study, our work demonstrated that TMP improved liver histological architecture, decreased hepatic enzyme levels and attenuated collagen deposition in the rat fibrotic liver. In addition, TMP significantly protected the liver from CCl4-caused injury and fibrogenesis by suppressing inflammation with reducing levels of inflammatory cytokines, including tumor necrosis factor-alpha (TNF-alpha), NLRP3, nuclear factor-kappa B (NF-kappaB) and interleukin-1beta (IL-1beta). Experiments in vitro showed that TMP inhibited inflammatory cytokine expression in HSCs associated with disrupting platelet-derived growth factor-b receptor (PDGF-betaR)/NLRP3/caspase1 pathway. These data collectively indicate that TMP can attenuate liver inflammation in liver fibrosis and possibly by targeting HSCs via PDGF-betaR/NLRP3/caspase1 pathway. It provides novel mechanistic insights into TMP as a potential therapeutic remedy for hepatic fibrosis.
Tetramethylpyrazine inhibits CoCl2 -induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1alpha/NOX2/ROS pathways.[Pubmed:25952107]
J Neurochem. 2015 Aug;134(3):551-65.
Hypoxia-mediated neurotoxicity contributes to various neurodegenerative disorders, including Alzheimer's disease and multiple sclerosis. Tetramethylpyrazine (TMP), a major bioactive component purified from Ligusticum wallichii Franchat, exhibited potent neuroprotective effect. However, the mechanism of TMP-exerted neuroprotective effect against hypoxia was not clear. In the study, we investigated the mechanism of the neuroprotective effect of TMP against hypoxia induced by CoCl2 in vitro and in vivo. The results showed that TMP could protect against CoCl2 -induced neurotoxicity in PC12 cells and in rats, as evidenced by enhancement of cell viability in PC12 cells and improvement of learning and memory ability in rats treated with CoCl2 . TMP could inhibit mitochondrial dysfunction, mitochondrial apoptotic molecular events, and thus apoptosis induced by CoCl2 . TMP inhibited CoCl2 -increased reactive oxygen species (ROS) level, which may contribute to hypoxia-related neurotoxicity induced by CoCl2 . The antioxidant and neuroprotective activities of TMP involved two pathways: one was the enhancement of nuclear factor erythroid 2-related factor 2 (Nrf2)/catalytic subunit of gamma-glutamylcysteine ligase-mediated regulation of GSH and the other was the inhibition of hypoxia-inducible factor 1 alpha/NADPH oxidase 2 (NOX2)-mediated ROS generation. These two pathways contributed to improvement of oxidative stress and thus the amelioration of apoptosis under hypoxic conditions. These results have appointed a new path toward the understanding of pathogenesis and TMP-related therapy of hypoxia-related neurodegenerative diseases. We proposed two cascades for Tetramethylpyrazine-exhibited protective effects against CoCl2 -induced neurotoxicity: One is enhancement of nuclear factor erythroid 2-related factor 2-catalytic subunit of gamma-glutamylcysteine ligase-mediated regulation of glutathone and the other was the inhibition of hypoxia-inducible factor 1 alpha-NADPH oxidase-2-mediated ROS generation. We think these findings should provide a new understanding of pathogenesis and Tetramethylpyrazine-related therapy of hypoxia-related neurodegenerative diseases.
Tetramethylpyrazine (TMP) exerts antitumor effects by inducing apoptosis and autophagy in hepatocellular carcinoma.[Pubmed:25841319]
Int Immunopharmacol. 2015 May;26(1):212-20.
Hepatocellular carcinoma (HCC) is one of the most common types of liver cancers with high recurrence rate and mortality rate. Recent studies have indicated that Tetramethylpyrazine (TMP), a purified chemical extracted from Ligusticum wallichii Franchat (ChuanXiong), possessed antitumor effects on HCC, but detailed mechanism remains unclear. Our study aims at investigating the antitumor effect of TMP on HCC and its underlying mechanism. We found that TMP inhibited cell proliferation of HepG2 cells in a dose-dependent way, and xenograft tumor models also indicated that high concentrations of TMP administration inhibited tumor growth. Next, flow cytometric analysis and transmission electron microscope images showed that TMP enhanced cell apoptosis in HepG2 cells, and western blot results showed that TMP promoted cleavage of caspase-3 and PARP in vitro and in vivo. We also found that TMP caused autophagy in HCC in vitro and in vivo. In order to examine the role of autophagy in TMP-induced apoptosis, 3-methyladenine (3-MA) was used to block the action of autophagy. Our data showed TMP-induced autophagy might be a pro-apoptosis process in HCC. Furthermore, the results of anti-oxidative enzymes and oxidation-sensitive fluorescent probe 2, 7-dichlorofluorescein diacetate (DCFH-DA) indicated that TMP induced ROS generation and inhibition of ROS diminished the anticancer function of TMP. In conclusion, our studies provide new insights into the mechanisms underlying the antitumor effect of TMP and suggest that TMP can be a novel therapeutic regimen for HCC.


