WAY-262611CAS# 1123231-07-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1123231-07-1 | SDF | Download SDF |
| PubChem ID | 25199517 | Appearance | Powder |
| Formula | C20H22N4 | M.Wt | 318.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 42 mg/mL (131.90 mM) *"≥" means soluble, but saturation unknown. | ||
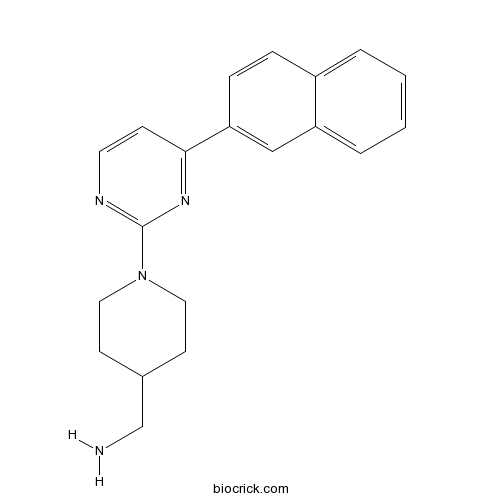
| Chemical Name | [1-(4-naphthalen-2-ylpyrimidin-2-yl)piperidin-4-yl]methanamine | ||
| SMILES | C1CN(CCC1CN)C2=NC=CC(=N2)C3=CC4=CC=CC=C4C=C3 | ||
| Standard InChIKey | QHLITPHIARVDJI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H22N4/c21-14-15-8-11-24(12-9-15)20-22-10-7-19(23-20)18-6-5-16-3-1-2-4-17(16)13-18/h1-7,10,13,15H,8-9,11-12,14,21H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

WAY-262611 Dilution Calculator

WAY-262611 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1405 mL | 15.7025 mL | 31.4051 mL | 62.8101 mL | 78.5127 mL |
| 5 mM | 0.6281 mL | 3.1405 mL | 6.281 mL | 12.562 mL | 15.7025 mL |
| 10 mM | 0.3141 mL | 1.5703 mL | 3.1405 mL | 6.281 mL | 7.8513 mL |
| 50 mM | 0.0628 mL | 0.3141 mL | 0.6281 mL | 1.2562 mL | 1.5703 mL |
| 100 mM | 0.0314 mL | 0.157 mL | 0.3141 mL | 0.6281 mL | 0.7851 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
WAY-262611 is a wingless β-Catenin agonist that increases bone formation rate with an EC50 of 0.63 μM in TCF-Luciferase assay.
In Vitro:WAY-262611 has the most potent activity in the primary assay, low kinase inhibition potential, and high solubility[1].
In Vivo:WAY-262611 has excellent pharmacokinetic properties and shows a dose dependent increase in the trabecular bone formation rate in ovariectomized rats following oral administration. Calvariae from wt mice treated with WAY-262611 shows statistically increased BFR, while similarly treated KO animals are no different from control. This indicates that WAY-262611 is acting via the Wnt β-catenin pathway and most likely through inhibition of Dkk-1[1].
References:
[1]. Pelletier JC, et al. (1-(4-(Naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine: a wingless beta-catenin agonist that increases bone formation rate. J Med Chem. 2009 Nov 26;52(22):6962-5.
- BRL 52537 hydrochloride
Catalog No.:BCC6751
CAS No.:112282-24-3
- H-Asp(OcHex)-OH
Catalog No.:BCC2887
CAS No.:112259-66-2
- 20(R)-Ginsenoside Rh2
Catalog No.:BCN2484
CAS No.:112246-15-8
- (S)-tert-Leucinol
Catalog No.:BCN8367
CAS No.:112245-13-3
- Stigmastane-3,6-diol
Catalog No.:BCN6003
CAS No.:112244-29-8
- 16-O-Methyl-14,15-didehydroisovincanol
Catalog No.:BCN1618
CAS No.:112237-71-5
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- DMAP
Catalog No.:BCC2842
CAS No.:1122-58-3
- Ikshusterol 3-O-glucoside
Catalog No.:BCN6001
CAS No.:112137-81-2
- (R)-(-)-Modafinic acid
Catalog No.:BCC5157
CAS No.:112111-45-2
- (S)-(+)-Modafinic acid
Catalog No.:BCC5158
CAS No.:112111-44-1
- DMPQ dihydrochloride
Catalog No.:BCC6977
CAS No.:1123491-15-5
- XL765
Catalog No.:BCC2060
CAS No.:1123889-87-1
- Tetramethylpyrazine
Catalog No.:BCN1008
CAS No.:1124-11-4
- 3-Deoxysappanchalcone
Catalog No.:BCN3736
CAS No.:112408-67-0
- 3'-Deoxy-4-O-methylsappanol
Catalog No.:BCN3675
CAS No.:112408-68-1
- 2',5,7-Trihydroxy-8-methoxyflavanone
Catalog No.:BCN6004
CAS No.:112408-71-6
- Ganoderic acid TN
Catalog No.:BCN2443
CAS No.:112430-64-5
- Ganoderic acid T-Q
Catalog No.:BCN3209
CAS No.:112430-66-7
- Ganoderic acid Jb
Catalog No.:BCN7972
CAS No.:112430-68-9
- AZ3146
Catalog No.:BCC3731
CAS No.:1124329-14-1
- 3-Phenyl-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN4005
CAS No.:112448-69-8
- U-54494A hydrochloride
Catalog No.:BCC6668
CAS No.:112465-94-8
Saikosaponin A Protects From Pressure Overload-Induced Cardiac Fibrosis via Inhibiting Fibroblast Activation or Endothelial Cell EndMT.[Pubmed:30443195]
Int J Biol Sci. 2018 Oct 31;14(13):1923-1934.
Saikosaponin A (SSA) is a triterpenoid saponin with many pharmacological activities, including anti-inflammatory and antioxidant effects. The effect of SSA on cardiac remodeling and fibrosis, however, remains unclear. Aortic banding surgery was used to establish a mouse cardiac remodeling and fibrosis model. Mice were subjected to an intraperitoneal (i.p.) injection of SSA (5 mg/kg/d or 40 mg/kg/d) 2 weeks after surgery for 28 days. As a result, SSA had limited effect on cardiac hypertrophy but decreased cardiac fibrosis remarkably. Neonatal rat cardiomyocytes were isolated and cultured with SSA (1 and 30 muM). Both 1 and 30 muM SSA reduced atrial natriuretic peptide transcription induced by angiotensin II. Adult mouse cardiac fibroblasts were isolated and cultured with SSA (1, 3, 10 and 30 muM). Only 10 and 30 muM SSA ameliorated transforming growth factor beta (TGFbeta)-induced fibroblast activation and function. Mouse heart endothelial cells were isolated and stimulated with TGFbeta and cocultured with SSA (1, 3, 10 and 30 muM). Only 1 and 3 muM SSA ameliorated TGFbeta-induced endothelium-mesenchymal transition (EndMT). Consistently, only the 5 mg/kg/d treatment relieved pressure overload-induced EndMT in vivo. Furthermore, we found that high dosages of SSA (10 and 30 muM) inhibited the TGFbeta/smad pathway in fibroblasts, while low dosages of SSA (1 and 3 muM) inhibited the Wnt/beta-catenin pathway in endothelial cells. The Smad pathway activator SRI-011381 eliminated SSA (30 muM)-induced protective effects on fibroblasts. The Wnt pathway activator WAY-262611 eliminated SSA (1 muM)-induced protective effects on endothelial cells. In summary, this study indicates the potential application of SSA in the treatment of myocardial fibrosis in cardiac fibrosis, with different target effects associated with different dosages.
Activation of dickkopf-1 and focal adhesion kinase pathway by tumour necrosis factor alpha induces enhanced migration of fibroblast-like synoviocytes in rheumatoid arthritis.[Pubmed:26715774]
Rheumatology (Oxford). 2016 May;55(5):928-38.
OBJECTIVE: The objective of this study was to investigate the roles of dickkopf-1 (DKK-1) and integrin-related focal adhesion kinase (FAK) by TNF-alpha on the migration of fibroblast-like synoviocytes (FLSs) in RA. METHODS: Wound scratch assays were performed to assess FLS migration. Western blotting was used to measure the levels of DKK-1, Wnt signalling molecules and FAK signalling molecules. Quantitative real-time PCR was used to measure the expression levels of DKK-1, integrin alphav, laminin, fibronectin, E-cadherin, MMP-8 and MMP-13. The concentrations of DKK-1, TNF-alpha and GSK-3beta were measured by ELISA. Genetic silencing of TNF-alpha was achieved by the transfection of small interfering RNA into cells. RESULTS: Migrating RA FLSs exhibited higher levels of DKK-1 and TNF-alpha expression compared with those in OA FLSs and/or stationary RA FLSs. Moreover, migrating FLSs exhibited significantly higher levels of FAK, p-JNK, paxillin and cdc42 expression, whereas the level of cytosolic beta-catenin was lower. WAY-262611, Wnt pathway agonist via inhibition of DKK-1, markedly inhibited cell migration of RA FLSs through the accumulation of cytosolic beta-catenin and suppression of FAK-related signalling pathways. TNF-alpha treatment to RA FLSs up-regulated expression of DKK-1, integrin alphav, fibronectin, laminin and MMP-13. TNF-alpha stimulation also suppressed cytosolic beta-catenin and E-cadherin expression in a time-dependent manner. Moreover, TNF-alpha small interfering RNA-transfected migrating FLSs exhibited decreased activation of integrin-related FAK, paxillin, p-JNK and cdc42 signalling pathways. CONCLUSION: This study demonstrates that the activation of DKK-1 and the integrin-related FAK signalling pathway stimulated by TNF-alpha induces the dissociation of beta-catenin/E-cadherin, thus promoting RA FLS migration.
Curcumin raises lipid content by Wnt pathway in hepatic stellate cell.[Pubmed:26414021]
J Surg Res. 2016 Feb;200(2):460-6.
BACKGROUND: Activation of hepatic stellate cells (HSCs) is a pivotal event in liver fibrosis, which is characterized by dramatic disappearance of lipid droplets. However, the underlying molecular mechanisms are largely unknown. We aimed to explore the role of Wnt/beta-catenin pathway in HSC lipogenesis and to examine the effects of curcumin in this molecular context. METHODS: Primary rat HSCs were cultured in vitro for experiments. The Wnt activator WAY-262611 and beta-catenin activator lithium chloride (LiCl) were used to activate the pathway at distinct levels in HSCs. Cell proliferation, fibrogenic markers, intracellular lipids and triglyceride, and adipogenic transcription factors were examined in HSCs. RESULTS: Both WAY-262611 and LiCl promoted proliferation and upregulated the expression of alpha-smooth muscle actin and alpha1(I) procollagen, but they decreased the contents of intracellular lipids and triglyceride in HSCs. Analyses of adipogenic transcription pattern showed that the two compounds reduced the expression of peroxisome proliferator-activated receptor gamma, CCAAT/enhancer binding protein alpha, retinoid X receptor-alpha, and retinoic acid receptor-beta, four key transcription regulators of HSC adipogenic phenotype. Curcumin also reduced the expression of Frizzled and beta-catenin, upregulated the expression of adipogenic transcription factors, and restored lipid content in HSCs. However, both WAY-262611 and LiCl abrogated curcumin restoration of lipogenesis and inhibition of fibrogenic marker expression in HSCs. CONCLUSIONS: Wnt/beta-catenin pathway was a profibrogenic signaling and inhibited lipogenesis by suppressing adipogenic transcription pattern in HSCs. Blockade of this pathway was associated with curcumin stimulation of HSC lipogenesis. We revealed a novel mechanism underlying curcumin restoration of lipid droplets during HSC activation.
GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition.[Pubmed:24561281]
Osteoarthritis Cartilage. 2014 Apr;22(4):566-77.
OBJECTIVE: Growth differentiation factor 5 (GDF5) is important for joint formation and associated with osteoarthritis (OA). Its role for the homeostasis of cartilage extracellular matrix (ECM) is, however, unknown. The canonical Wnt signaling pathway is also implemented in OA and activation of the pathway has detrimental effects on the cartilage ECM. The objective of this study was to investigate the effect of GDF5 stimulation on the Wnt signaling pathway and on the expression of known modulators of cartilage ECM. DESIGN: Human chondrocytes were cultured in the pellet mass system and stimulated with increasing concentrations of GDF5. Expression of matrix modulating enzymes and canonical Wnt inhibitors dickkopf 1 (DKK1) and frizzled related protein (FRZB) were measured with quantitative PCR (qPCR). Protein levels of matrix metalloprotease 13 (MMP13), DKK1 and beta-catenin were measured with enzyme-linked immunosorbent assay (ELISA). Canonical Wnt signaling was stimulated with Wnt3a and small molecule CHIR-99021 and DKK1 was blocked with small molecule WAY-262611. RESULTS: In this study, we show that GDF5 stimulation of human chondrocytes inhibits expression of the cartilage ECM degrading enzymes MMP13 and ADAMTS4 and stimulates the expression of cartilage anabolic genes ACAN and SOX9. We further show that the stimulation inhibits the canonical Wnt signaling pathway through expression of the canonical Wnt inhibitors DKK1 and FRZB. Finally we show that inhibition of MMP13 expression through GDF5 stimulation is mediated by DKK1. CONCLUSION: Herein, we provide evidence of a previously unknown link between GDF5 signaling and canonical Wnt signaling that may contribute to the understanding of the molecular mechanisms of OA.
(1-(4-(Naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine: a wingless beta-catenin agonist that increases bone formation rate.[Pubmed:19856966]
J Med Chem. 2009 Nov 26;52(22):6962-5.
A high-throughput screening campaign to discover small molecule leads for the treatment of bone disorders concluded with the discovery of a compound with a 2-aminopyrimidine template that targeted the Wnt beta-catenin cellular messaging system. Hit-to-lead in vitro optimization for target activity and molecular properties led to the discovery of (1-(4-(naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine (5, WAY-262611). Compound 5 has excellent pharmacokinetic properties and showed a dose dependent increase in the trabecular bone formation rate in ovariectomized rats following oral administration.


