3-DeoxysappanchalconeCAS# 112408-67-0 |
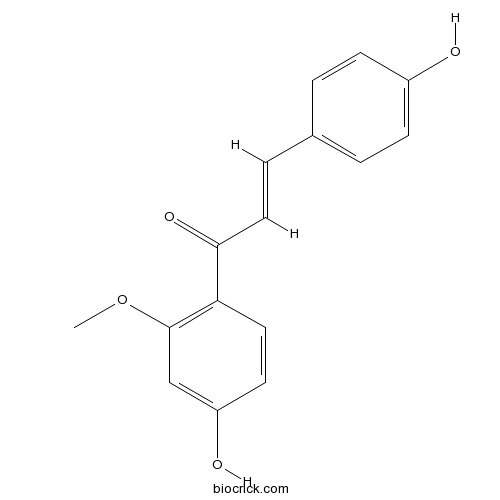
- 2'-O-Methylisoliquiritigenin
Catalog No.:BCN0657
CAS No.:51828-10-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112408-67-0 | SDF | Download SDF |
| PubChem ID | 5319688 | Appearance | Powder |
| Formula | C16H14O4 | M.Wt | 270.3 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Synonyms | 2'-O-Methylisoliquiritigenin;51828-10-5 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-1-(4-hydroxy-2-methoxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | COC1=C(C=CC(=C1)O)C(=O)C=CC2=CC=C(C=C2)O | ||
| Standard InChIKey | PACBGANPVNHGNP-RUDMXATFSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 3-Deoxysappanchalcone is an effective HO-1 inducer at the translational level. 2. 3-Deoxysappanchalcone has anti-inflammatory effects, is a valuable compound for modulating inflammatory conditions. 3. 3-Deoxysappanchalcone has anti-influenza virus activity, the mechanism involved anti-apoptosis and anti-inflammation activities in vitro. 4. 3-Deoxysappanchalcone has inhibitory activity on MMP-9 expression and production in TNF-α-treated cells, is mediated by the suppression of AP-1 and NF-κB activation. |
| Targets | HO-1 | mTOR | Akt | NO | IL Receptor | TNF-α | AP-1 | NF-kB | MMP(e.g.TIMP) |

3-Deoxysappanchalcone Dilution Calculator

3-Deoxysappanchalcone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6996 mL | 18.498 mL | 36.9959 mL | 73.9919 mL | 92.4898 mL |
| 5 mM | 0.7399 mL | 3.6996 mL | 7.3992 mL | 14.7984 mL | 18.498 mL |
| 10 mM | 0.37 mL | 1.8498 mL | 3.6996 mL | 7.3992 mL | 9.249 mL |
| 50 mM | 0.074 mL | 0.37 mL | 0.7399 mL | 1.4798 mL | 1.8498 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.7399 mL | 0.9249 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tetramethylpyrazine
Catalog No.:BCN1008
CAS No.:1124-11-4
- XL765
Catalog No.:BCC2060
CAS No.:1123889-87-1
- DMPQ dihydrochloride
Catalog No.:BCC6977
CAS No.:1123491-15-5
- WAY-262611
Catalog No.:BCC5507
CAS No.:1123231-07-1
- BRL 52537 hydrochloride
Catalog No.:BCC6751
CAS No.:112282-24-3
- H-Asp(OcHex)-OH
Catalog No.:BCC2887
CAS No.:112259-66-2
- 20(R)-Ginsenoside Rh2
Catalog No.:BCN2484
CAS No.:112246-15-8
- (S)-tert-Leucinol
Catalog No.:BCN8367
CAS No.:112245-13-3
- Stigmastane-3,6-diol
Catalog No.:BCN6003
CAS No.:112244-29-8
- 16-O-Methyl-14,15-didehydroisovincanol
Catalog No.:BCN1618
CAS No.:112237-71-5
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- 3'-Deoxy-4-O-methylsappanol
Catalog No.:BCN3675
CAS No.:112408-68-1
- 2',5,7-Trihydroxy-8-methoxyflavanone
Catalog No.:BCN6004
CAS No.:112408-71-6
- Ganoderic acid TN
Catalog No.:BCN2443
CAS No.:112430-64-5
- Ganoderic acid T-Q
Catalog No.:BCN3209
CAS No.:112430-66-7
- Ganoderic acid Jb
Catalog No.:BCN7972
CAS No.:112430-68-9
- AZ3146
Catalog No.:BCC3731
CAS No.:1124329-14-1
- 3-Phenyl-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN4005
CAS No.:112448-69-8
- U-54494A hydrochloride
Catalog No.:BCC6668
CAS No.:112465-94-8
- H-Glu(OcHex)-OH
Catalog No.:BCC2929
CAS No.:112471-82-6
- 4,4-Pentamethylenepiperidine hydrochloride
Catalog No.:BCC6059
CAS No.:1125-01-5
- 5-Aminoisoquinoline
Catalog No.:BCC8736
CAS No.:1125-60-6
- 6-Aldehydo-isoophiopogonone A
Catalog No.:BCN6629
CAS No.:112500-90-0
3-Deoxysappanchalcone inhibits tumor necrosis factor-alpha-induced matrix metalloproteinase-9 expression in human keratinocytes through activated protein-1 inhibition and nuclear factor-kappa B DNA binding activity.[Pubmed:21628889]
Biol Pharm Bull. 2011;34(6):890-3.
Tumor necrosis factor alpha (TNF-alpha), which is a primary cytokine responsible for inflammatory responses in skin, induces the synthesis of matrix metalloproteinase-9 (MMP-9), which causes skin aging. The protective effects of 3-Deoxysappanchalcone against TNF-alpha-induced damage was investigated using human skin keratinocytes. The results showed that 3-Deoxysappanchalcone inhibited MMP-9 expression at the protein and mRNA level, by blocking the activation of activator protein-1 (AP-1) and nuclear factor kappa B (NF-kappaB). Taken together, the inhibitory activity of 3-Deoxysappanchalcone on MMP-9 expression and production in TNF-alpha-treated cells was found to be mediated by the suppression of AP-1 and NF-kappaB activation.
Sedative-hypnotic and anxiolytic effects and the mechanism of action of aqueous extracts of peanut stems and leaves in mice.[Pubmed:29572847]
J Sci Food Agric. 2018 Oct;98(13):4885-4894.
INTRODUCTION: Peanut stems and leaves (PSL) have traditionally been used as both a special food and a herbal medicine in Asia. The sedative-hypnotic and anxiolytic effects of PSL have been recorded in classical traditional Chinese literature, and more recently by many other researchers. In a previous study, four sleep-related ingredients (linalool, 5-hydroxy-4',7-dimethoxyflavanone, 2'-O-methylisoliquiritigenin and ferulic acid), among which 5-hydroxy-4',7-dimethoxyflavanone and 2'-O-methylisoliquiritigenin were newly found in Arachis species, were screened by ultrahigh-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC/QTOF-MS). In the current study, quantitative examination of the above four ingredients was conducted. Serious fundamental functional studies were done in mice, including locomotor activity, direct sleep tests, pentobarbital-induced sleeping time tests, subthreshold dose of pentobarbital tests and barbital sodium sleep incubation period tests, to determine the material base for the sedative-hypnotic and anxiolytic effects of aqueous extracts of PSL. Furthermore, neurotransmitter levels in three brain regions (cerebrum, cerebellum and brain stem) were determined using UHPLC coupled with triple-quadrupole mass spectrometry (UHPLC/QQQ-MS) in order to elucidate the exact mechanism of action. RESULTS: Aqueous extract of PSL at a dose of 500 mg kg(-1) (based on previous experience), along with different concentrations of the above four functional ingredients (189.86 microg kg(-1) linalool, 114.75 mg kg(-1) 5-hydroxy-4',7-dimethoxyflavanone, 32.4mg kg(-1) 2'-O-methylisoliquiritigenin and 44.44 mg kg(-1) ferulic acid), had a sedative-hypnotic effect by affecting neurotransmitter levels in mice. CONCLUSION: The data demonstrate that these four ingredients are the key functional factors for the sedative-hypnotic and anxiolytic effects of PSL aqueous extracts and that these effects occur via changes in neurotransmitter levels and pathways. (c) 2018 Society of Chemical Industry.
The anti-inflammatory effect of 3-deoxysappanchalcone is mediated by inducing heme oxygenase-1 via activating the AKT/mTOR pathway in murine macrophages.[Pubmed:25091623]
Int Immunopharmacol. 2014 Oct;22(2):420-6.
3-Deoxysappanchalcone (3-DSC), isolated from Caesalpinia sappan (Leguminosae), is a chalcone that exerts a variety of pharmacological activities. In the present study, we demonstrated that 3-DSC exerts anti-inflammatory activity in murine macrophages by inducing heme oxygenase-1 (HO-1) expression at the translational level. Treatment of RAW264.7 cells with 3-DSC induced HO-1 protein expression in a dose- and time-dependent manner without affecting HO-1 mRNA expression. Mitogen-activated protein kinase inhibitors or actinomycin D, a transcriptional inhibitor, did not block 3-DSC-mediated HO-1 induction. However, 3-DSC-mediated HO-1 induction was completely blocked by treatment with cycloheximide, a translational inhibitor, or rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR). Strikingly, 3-DSC increased the phosphorylation level of mTOR downstream target molecules such as eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1), as well as AKT in a dose- and time-dependent manner, suggesting that the 3-DSC induces HO-1 expression by activating the AKT/mTOR pathway. Consistent with the notion that HO-1 has anti-inflammatory properties, 3-DSC inhibited the production of nitric oxide (NO) and interleukin (IL)-6 in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Inhibition of HO-1 activity by treatment with tin protoporphyrin IX, a specific HO-1 inhibitor, abrogated the inhibitory effects of 3-DSC on the production of NO and IL-6 in LPS-stimulated RAW264.7 cells. Taken together, 3-DSC may be an effective HO-1 inducer at the translational level that has anti-inflammatory effects, and a valuable compound for modulating inflammatory conditions.
The protective effect of 3-deoxysappanchalcone on in vitro influenza virus-induced apoptosis and inflammation.[Pubmed:22648377]
Planta Med. 2012 Jun;78(10):968-73.
Influenza virus is one of the most important causes of acute respiratory disease. Viral infection and viral replication activate multiple cell signalling pathways. Apoptosis of infected cells and immune response against viral replication, which are generally considered to be protective mechanisms, are also probably mediated by viruses, which lead to severe health problems. We previously reported that 3-Deoxysappanchalcone (3-DSC), a compound that is isolated from Caesalpinia sappan, exhibited in vitro anti-influenza activity. In the present study, we further identified that 3-DSC inhibited viral genomic replication and transcription only at a relatively high concentration. We then evaluated the effect of 3-DSC on the regulation of virus-induced cellular apoptosis. 3-DSC ameliorated virus-induced DNA fragmentation in a concentration-dependent manner, which tends to be a consequence of its inhibition of upstream caspase activation. 3-DSC also protected host cells against influenza-induced inflammation by suppressing CCL5 and CXCL10 secretions in endothelial cells and reducing the production of IL-6 and IL-1beta in monocytes/macrophages. In conclusion, our results demonstrate that anti-influenza virus mechanisms of 3-DSC involved anti-apoptosis and anti-inflammation activities in vitro. Moreover, 3-DSC could be a promising drug candidate for influenza treatment.


