KC7F2HIF-1α inhibitor CAS# 927822-86-4 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- (±)-Bay K 8644
Catalog No.:BCC3918
CAS No.:71145-03-4
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 927822-86-4 | SDF | Download SDF |
| PubChem ID | 16047442 | Appearance | Powder |
| Formula | C16H16Cl4N2O4S4 | M.Wt | 570.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 32 mg/mL (56.10 mM) *"≥" means soluble, but saturation unknown. | ||
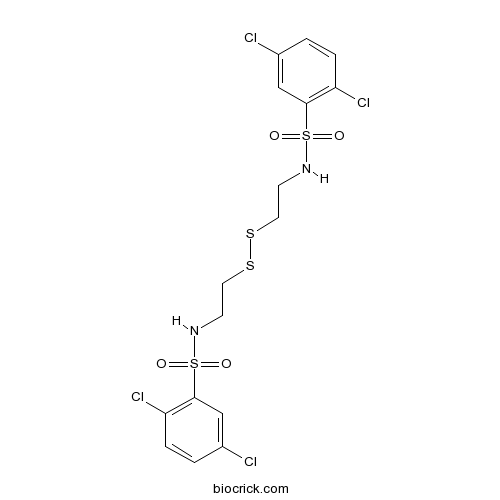
| Chemical Name | 2,5-dichloro-N-[2-[2-[(2,5-dichlorophenyl)sulfonylamino]ethyldisulfanyl]ethyl]benzenesulfonamide | ||
| SMILES | C1=CC(=C(C=C1Cl)S(=O)(=O)NCCSSCCNS(=O)(=O)C2=C(C=CC(=C2)Cl)Cl)Cl | ||
| Standard InChIKey | REQLACDIZMLXIC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16Cl4N2O4S4/c17-11-1-3-13(19)15(9-11)29(23,24)21-5-7-27-28-8-6-22-30(25,26)16-10-12(18)2-4-14(16)20/h1-4,9-10,21-22H,5-8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of HIF-1α. Thought to act via down-regulation of HIF-1α protein synthesis; reduces phosphorylation of eIF4E binding protein 1 (4EBP1) and p70 S6K in hypoxic conditions. Also blocks hypoxia-induced HIF-1α accumulation in a range of human cancer cell lines. Inhibits the expression of HIF target genes, such as carbonic anhydrase IX and MMP2. |

KC7F2 Dilution Calculator

KC7F2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7532 mL | 8.7661 mL | 17.5322 mL | 35.0643 mL | 43.8304 mL |
| 5 mM | 0.3506 mL | 1.7532 mL | 3.5064 mL | 7.0129 mL | 8.7661 mL |
| 10 mM | 0.1753 mL | 0.8766 mL | 1.7532 mL | 3.5064 mL | 4.383 mL |
| 50 mM | 0.0351 mL | 0.1753 mL | 0.3506 mL | 0.7013 mL | 0.8766 mL |
| 100 mM | 0.0175 mL | 0.0877 mL | 0.1753 mL | 0.3506 mL | 0.4383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KC7F2 is a novel inhibitor of HIF-1αwith IC50 value of 20 μM [1].
Hypoxia inducible factor-1 (HIF-1) is a heterodimeric transcription factor consisting of α and β subunits. In normal situation, the HIF-1α subunit is constitutively translated, but rapidly degraded. While, under hypoxia it is stabilized. HIF target genes encode a series of critical factors to adapt the low oxygen [1].
In a HIF-reporter cell line LN229-HRE-AP, KC7F2 (>25 μM) reduced AP activity by 90% in LN229 cells, which indicated that KC7F2 inhibited AP enzyme activity. In LNZ308 human glioma cells, KC7F2 inhibited the expression of a panel of HIF target genes, such as matrix metalloproteinase 2 (MMP2), enolase 1, carbonic anhydrase IX (CA IX) and endothelin 1. Also, KC7F2 reduced expression of HIF-1α in a dose-dependent way [1].
In a rat epilepsy model, KC7F2 significantly shortened the latent period in the PTZ kindling model and increased the rate of spontaneous recurrent seizures during the chronic stage in the lithium-pilocarpine model [3].
References:
[1]. Narita T, Yin S, Gelin CF, et al. Identification of a novel small molecule HIF-1alpha translation inhibitor. Clin Cancer Res, 2009, 15(19): 6128-6136.
[2]. Li J, Jiang G, Chen Y, et al. Altered expression of hypoxia-Inducible factor-1α participates in the epileptogenesis in animal models. Synapse, 2014, 68(9): 402-409.
- Glochicoccin D
Catalog No.:BCN4471
CAS No.:927812-23-5
- Motolimod (VTX-2337)
Catalog No.:BCC6497
CAS No.:926927-61-9
- Toll-like receptor modulator
Catalog No.:BCC2007
CAS No.:926927-42-6
- Germacrone 4,5-epoxide
Catalog No.:BCN6724
CAS No.:92691-35-5
- Deltatsine
Catalog No.:BCN8106
CAS No.:92631-66-8
- GGsTop
Catalog No.:BCC6187
CAS No.:926281-37-0
- BG45
Catalog No.:BCC6469
CAS No.:926259-99-6
- Milnacipran
Catalog No.:BCC4194
CAS No.:92623-85-3
- O-Demethylforbexanthone
Catalog No.:BCN4469
CAS No.:92609-77-3
- Radotinib(IY-5511)
Catalog No.:BCC6398
CAS No.:926037-48-1
- AMTB hydrochloride
Catalog No.:BCC7834
CAS No.:926023-82-7
- Dayecrystal A
Catalog No.:BCN4859
CAS No.:926010-24-4
- DPNI-caged-GABA
Catalog No.:BCC5957
CAS No.:927866-58-8
- RAF265
Catalog No.:BCC3677
CAS No.:927880-90-8
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- Alisol O
Catalog No.:BCN3362
CAS No.:928148-51-0
- Tenacissoside F
Catalog No.:BCN4472
CAS No.:928151-78-4
- 3-Chloro-1-(4-octylphenyl)-propanone
Catalog No.:BCN2249
CAS No.:928165-59-7
- Boc-D-Asp-OBzl
Catalog No.:BCC3370
CAS No.:92828-64-3
- MN 64
Catalog No.:BCC6489
CAS No.:92831-11-3
- AS 1892802
Catalog No.:BCC6335
CAS No.:928320-12-1
- IRAK inhibitor 2
Catalog No.:BCC1655
CAS No.:928333-30-6
- PF-03716556
Catalog No.:BCC2084
CAS No.:928774-43-0
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
Folic Acid Represses Hypoxia-Induced Inflammation in THP-1 Cells through Inhibition of the PI3K/Akt/HIF-1alpha Pathway.[Pubmed:26974319]
PLoS One. 2016 Mar 14;11(3):e0151553.
Though hypoxia has been implicated as a cause of inflammation, the underlying mechanism is not well understood. Folic acid has been shown to provide protection against oxidative stress and inflammation in patients with cardiovascular disease and various models approximating insult to tissue via inflammation. It has been reported that hypoxia-induced inflammation is associated with oxidative stress, upregulation of hypoxia-inducible factor 1-alpha (HIF-1alpha), and production of pro-inflammatory molecules. Whether folic acid protects human monocytic cells (THP-1 cells) against hypoxia-induced damage, however, remains unknown. We used THP-1 cells to establish a hypoxia-induced cellular injury model. Pretreating THP-1 cells with folic acid attenuated hypoxia-induced inflammatory responses, including a decrease in protein and mRNA levels of interleukin (IL)-1beta and tumor necrosis factor-alpha (TNF-alpha), coupled with increased levels of IL-10. Folic acid also reduced hypoxia-induced Akt phosphorylation and decreased nuclear accumulation of HIF-1alpha protein. Both LY294002 (a selective inhibitor of phosphatidyl inositol-3 kinase, PI3K) and KC7F2 (a HIF-1alpha inhibitor) reduced levels of hypoxia-induced inflammatory cytokines. We also found that insulin (an Akt activator) and dimethyloxallyl glycine (DMOG, a HIF-1alpha activator) induced over-expression of inflammatory cytokines, which could be blocked by folic acid. Taken together, these findings demonstrate how folic acid attenuates the hypoxia-induced inflammatory responses of THP-1 cells through inhibition of the PI3K/Akt/HIF-1alpha pathway.
The HIF-1alpha/CXCR4 pathway supports hypoxia-induced metastasis of human osteosarcoma cells.[Pubmed:25444927]
Cancer Lett. 2015 Feb 1;357(1):254-264.
HIF-1alpha mediates hypoxia-induced expression of the chemokine receptor CXCR4 and contributes to metastasis in many different cancers. We have previously shown that hypoxia promotes migration of human osteosarcoma cells by activating the HIF-1alpha/CXCR4 pathway. Here, immunohistochemical analysis showed that unlike control osteochondroma samples, osteosarcoma specimens were characterized by elevated expression levels of HIF-1alpha and CXCR4. Moreover, we found that hypoxia-induced invasiveness was more pronounced in high metastatic potential F5M2 osteosarcoma cells than in low metastatic potential F4 cells, and that this induction was sensitive to treatment with the CXCR4 antagonist AMD3100 and the HIF-1alpha inhibitor KC7F2. Interestingly, hypoxia-induced CXCR4 expression persisted after cultured osteosarcoma cells were returned to normoxic conditions. These observations were confirmed by experiments in a mouse model of osteosarcoma lung metastasis showing that hypoxia stimulation of pulmonary metastasis was greater in F5M2 than in F4 cells, and was sensitive to treatment with AMD3100. Our study provides further evidence of the contributions of hypoxia and the HIF-1alpha/CXCR4 pathway to the progression of osteosarcoma, and suggests that this axis might be efficiently leveraged in the development of novel osteosarcoma therapeutics.
Inhibition of hypoxia inducible factor-1alpha downregulates the expression of epithelial to mesenchymal transition early marker proteins without undermining cell survival in hypoxic lens epithelial cells.[Pubmed:26392741]
Mol Vis. 2015 Sep 1;21:1024-35. eCollection 2015.
PURPOSE: The purpose of this study was to identify potential therapeutic strategies to slow down or prevent the expression of early-onset epithelial to mesenchymal transition (EMT) marker proteins (fibronectin and alpha smooth muscle actin, alpha-SMA) without sacrificing the synthesis and accumulation of the prosurvival protein vascular endothelial growth factor (VEGF) in cultured virally transformed human lens epithelial (HLE) cells. METHODS: HLE-B3 cells, maintained in a continuous hypoxic environment (1% oxygen), were treated with SB216763, a specific inhibitor of glycogen synthase kinase-3beta (GSK-3beta) catalytic activity. Western blot analysis was employed to detect the cytoplasmic and nuclear levels of beta-catenin, as well as the total lysate content of fibronectin and alpha-SMA. Enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of VEGF in cell culture medium. A hypoxia-inducible factor-1alpha (HIF-1alpha) translation inhibitor and an HIF-2alpha translation inhibitor were independently employed to evaluate the effect of hypoxia inducible factor inhibition on EMT marker protein and VEGF expression. XAV932 was used to assess the suppression of nuclear beta-catenin and its downstream effect on EMT marker proteins and VEGF expression. RESULTS: SB216763-treated HLE-B3 cells caused marked inhibition of GSK-3beta activity prompting a significant increase in the translocation of cytoplasmic beta-catenin to the nucleus. The enhancement of nuclear beta-catenin looked as if it positively correlated with a significant increase in the basal expression of VEGF as well as increased expression of fibronectin and alpha-SMA. In conjunction with SB216763, coadministration of an HIF-1alpha translation inhibitor, but not an HIF-2alpha translation inhibitor, markedly suppressed the expression of fibronectin and alpha-SMA without affecting VEGF levels. Treatment with XAV932 significantly reduced the level of nuclear beta-catenin, but the levels of neither the EMT marker proteins nor VEGF were changed. CONCLUSIONS: Recently, we reported that nuclear beta-catenin, but not HIF-2alpha, regulates the expression of fibronectin and alpha-SMA in atmospheric oxygen. In marked contrast, data from the hypoxic condition clearly establish that nuclear beta-catenin plays little apparent role in the expression of EMT marker proteins. Instead, the loss of HIF-1alpha (but not HIF-2alpha) decreases the expression of the EMT marker proteins without sacrificing the levels of the prosurvival protein VEGF. These findings support the development of a potentially relevant therapeutic strategy to undermine the progression of normal cells to the mesenchymal phenotype in the naturally hypoxic lens without subverting cell viability.
Hypoxia induces the dysfunction of human endothelial colony-forming cells via HIF-1alpha signaling.[Pubmed:28964937]
Respir Physiol Neurobiol. 2018 Jan;247:87-95.
Endothelial injury is considered as a trigger of pulmonary vascular lesions in the pathogenesis of hypoxic pulmonary hypertension (HPH). Although endothelial colony-forming cells (ECFCs) have vascular regeneration potential to maintain endothelial integrity, hypoxia-induced precise alteration in ECFCs function remains controversial. This study investigated the impact of hypoxia on human ECFCs function in vitro and the underlying mechanism. We found that hypoxia inhibited ECFCs proliferation, migration and angiogenesis. Compared with no treatment, the expression of hypoxia inducible factor-1alpha (HIF-1alpha) in hypoxia-treated ECFCs was increased, with an up-regulation of p27 and a down-regulation of cyclin D1. The over-secreted vascular endothelial growth factor (VEGF) was detected, with the imbalanced expression of fetal liver kinase 1 (flk-1) and fms related tyrosine kinase 1 (flt-1). Hypoxia-induced changes in ECFCs could be reversed by HIF-1alpha inhibitor KC7F2. These data suggest that HIF-1alpha holds the key in regulating ECFCs function which may open a new perspective of ECFCs in HPH management.
Blocking heme oxygenase-1 by zinc protoporphyrin reduces tumor hypoxia-mediated VEGF release and inhibits tumor angiogenesis as a potential therapeutic agent against colorectal cancer.[Pubmed:26822586]
J Biomed Sci. 2016 Jan 28;23:18.
BACKGROUND: Hypoxia in tumor niche is one of important factors to start regeneration of blood vessels, leading to increase survival, proliferation, and invasion in cancer cells. Under hypoxia microenvironment, furthermore, steadily increased hypoxia-inducible factor -1alpha (HIF-1alpha) is observed, and can increase vascular endothelial growth factor (VEGF) expression and promote angiogenesis. Zinc protoporphyrin (ZnPP), a heme oxygenase-1 (HO-1) inhibitor, is potential to inhibit tumor proliferation and progression. However, the mechanism of ZnPP in inhibition of tumor is not completely clear. We hypothesize that ZnPP may modulate HIF-1alpha through inhibiting HO-1, and then inhibit angiogenesis and tumor progression. This study aimed to dissect the mechanism of ZnPP in tumor suppression. RESULTS: We observed the amount of VEGF was increased in the sera of the colorectal cancer (CRC) patients (n = 34, p < 0.05). Furthermore, increased VEGF expression was also measured in colorectal cancer cells, HCT-15, culturing under mimicking hypoxic condition. It suggested that hypoxia induced VEGF production from cancer cells. VEGF production was significantly reduced from HCT-15 cells after exposure to HIF-1alpha inhibitor KC7F2, suggesting that HIF-1alpha regulated VEGF production. Moreover, we observed that the HO-1 inhibitor ZnPP inhibited the expressions of HIF-1alpha and VEGF coupled with cell proliferations of HCT-15 cells, suggesting that ZnPP blocked HIF-1alpha expression, and then inhibited the consequent VEGF production. In the xenograft model, we also observed that the animals exposed to ZnPP displayed much smaller tumor nodules and less degree of angiogenesis with decreased expression of the angiogenesis marker, alphavbeta3 integrin, compared to that in normal control. CONCLUSIONS: This study demonstrated that VEGF level in serum was elevated in the patients with CRC. The HO-1 inhibitor, ZnPP, possessed the properties of anti-tumor agent by decreasing HIF-1alpha levels, blocking VEGF production, impairing tumor angiogenesis, and inhibiting tumor growth.
Inhibiting the hypoxia response for cancer therapy: the new kid on the block.[Pubmed:19789327]
Clin Cancer Res. 2009 Oct 1;15(19):5945-6.
The hypoxia-inducible transcription factor (HIF)-1alpha inhibitor KC7F2 described in this issue of Clinical Cancer Research is the newest addition to an emerging class of antitumor agents targeting the hypoxia response. Here, we discuss the proposed mechanism of action of KC7F2 and its potential strengths and limitations in comparison with other promising HIF-1alpha inhibitors.
Identification of a novel small molecule HIF-1alpha translation inhibitor.[Pubmed:19789328]
Clin Cancer Res. 2009 Oct 1;15(19):6128-36.
PURPOSE: Hypoxia inducible factor-1 (HIF-1), the central mediator of the cellular response to low oxygen, functions as a transcription factor for a broad range of genes that provide adaptive responses to oxygen deprivation. HIF-1 is overexpressed in cancer and has become an important therapeutic target in solid tumors. In this study, a novel HIF-1alpha inhibitor was identified and its molecular mechanism was investigated. EXPERIMENTAL DESIGN: Using a HIF-responsive reporter cell-based assay, a 10,000-member natural product-like chemical compound library was screened to identify novel HIF-1 inhibitors. This led us to discover KC7F2, a lead compound with a central structure of cystamine. The effects of KC7F2 on HIF-1 transcription, translation, and protein degradation processes were analyzed. RESULTS: KC7F2 markedly inhibited HIF-mediated transcription in cells derived from different tumor types, including glioma, breast, and prostate cancers, and exhibited enhanced cytotoxicity under hypoxia. KC7F2 prevented the activation of HIF-target genes such as carbonic anhydrase IX, matrix metalloproteinase 2 (MMP2), endothelin 1, and enolase 1. An investigation into the mechanism of action of KC7F2 showed that it worked through the down-regulation of HIF-1alpha protein synthesis, an effect accompanied by the suppression of the phosphorylation of eukaryotic translation initiation factor 4E binding protein 1 and p70 S6 kinase, key regulators of HIF-1alpha protein synthesis. CONCLUSION: These results show that KC7F2 is a potent HIF-1 pathway inhibitor and its potential as a cancer therapy agent warrants further study.


