Rocilinostat (ACY-1215)Selective HDAC6 inhibitor CAS# 1316214-52-4 |
- Tubastatin A
Catalog No.:BCC2158
CAS No.:1252003-15-8
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- Nexturastat A
Catalog No.:BCC5345
CAS No.:1403783-31-2
- Tubacin
Catalog No.:BCC2428
CAS No.:537049-40-4
- Resminostat (RAS2410)
Catalog No.:BCC2165
CAS No.:864814-88-0
- TCS HDAC6 20b
Catalog No.:BCC2427
CAS No.:956154-63-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1316214-52-4 | SDF | Download SDF |
| PubChem ID | 53340666 | Appearance | Powder |
| Formula | C24H27N5O3 | M.Wt | 433.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ricolinostat; Rocilinostat | ||
| Solubility | DMSO : ≥ 50 mg/mL (115.34 mM) *"≥" means soluble, but saturation unknown. | ||
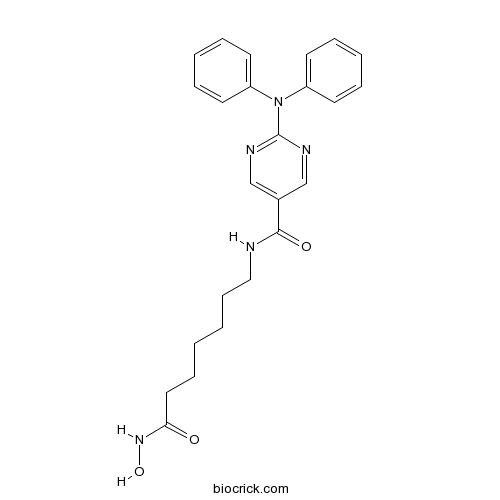
| Chemical Name | N-[7-(hydroxyamino)-7-oxoheptyl]-2-(N-phenylanilino)pyrimidine-5-carboxamide | ||
| SMILES | C1=CC=C(C=C1)N(C2=CC=CC=C2)C3=NC=C(C=N3)C(=O)NCCCCCCC(=O)NO | ||
| Standard InChIKey | QGZYDVAGYRLSKP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rocilinostat (ACY-1215) is a selective inhibitor of HDAC6 with IC50 of 5 nM. | |||||
| Targets | HDAC6 | |||||
| IC50 | 5 nM | |||||
| Kinase experiment [1]: | |
| Inhibitory activities | ACY-1215 was dissolved and subsequently diluted in assay buffer [50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, and 20 μM tris(2-carboxyethyl)phosphine] to 6-fold the final concentration. HDAC enzymes were diluted to 1.5-fold of the final concentration in assay buffer and pre-incubated with ACY-1215 for 10 minutes before the addition of the substrate. The amount of FTS (HDAC1, HDAC2, HDAC3, and HDAC6) or MAZ-1675 (HDAC4, HDAC5, HDAC7, HDAC8, and HDAC9) used for each enzyme was equal to the Michaelis constant (Km), as determined by a titration curve. FTS or MAZ-1675 was diluted in assay buffer to 6-fold the final concentration with 0.3 μM sequencing grade trypsin. The substrate/trypsin mix was added to the enzyme/compound mix and the plate was shaken for 60 seconds and then placed into a SpectraMax M5 microtiter plate reader. The enzymatic reaction was monitored for release of 7-amino-4-methoxy-coumarin over 30 minutes, after deacetylation of the lysine side chain in the peptide substrate, and the linear rate of the reaction was calculated. HDAC11, sirtuin1, and sirtuin2 assays were performed by Cerep. |
| Cell experiment [1]: | |
| Cell lines | MM cells; PBMCs. |
| Preparation method | Soluble in DMSO > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 0.16, 0.8, 4, 20 nM; MM cells: 24 h, PBMCs: 48 h. |
| Applications | ACY-1215 induces less cytotoxicity in PHA-stimulated or unstimulated PBMCs. In purified CD4+ T cells, ACY-1215 induces toxicity with IC50 value of 2.5 μM. In MM cell lines, ACY-1215 dose-dependently decreases viability with IC50 value of 2-8 μM and induces significant cytotoxicity. In MM.1S cells, ACY-1215 dose-dependently reduces DNA synthesis of MM cells adherent to BMSCs and also inhibits growth induced by IL-6 and IGF-1. |
| Animal experiment [1]: | |
| Animal models | Male SCID mice inoculated subcutaneously with MM.1S cells. |
| Dosage form | 50 mg/kg; 5 days a week for 3 weeks; administrated orally. |
| Preparation method | Dissolved in 10% DMSO in 5% dextrose in water |
| Application | In human MM xenograft mouse model, ACY-1215 (50 mg/kg) significantly delays tumor growth. Treatment mice with ACY-1215 plus bortezomib significantly prolonged overall survival (OS) and induces a significant accumulation of polyubiquitinated proteins. Treatment with ACY-1215 plus bortezomib is well tolerated and have no significant influence on the body weight. In female SCID-beige mice inoculated intravenously with MM.1S-LucNeo cells, treatment with ACY-1215 (75 mg/kg) and bortezomib (1.5 mg/kg) significantly inhibits tumor growth and prolongs OS. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1]. Santo L, Hideshima T, Kung AL, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood, 2012, 119(11): 2579-2589. | |

Rocilinostat (ACY-1215) Dilution Calculator

Rocilinostat (ACY-1215) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3068 mL | 11.534 mL | 23.0681 mL | 46.1361 mL | 57.6701 mL |
| 5 mM | 0.4614 mL | 2.3068 mL | 4.6136 mL | 9.2272 mL | 11.534 mL |
| 10 mM | 0.2307 mL | 1.1534 mL | 2.3068 mL | 4.6136 mL | 5.767 mL |
| 50 mM | 0.0461 mL | 0.2307 mL | 0.4614 mL | 0.9227 mL | 1.1534 mL |
| 100 mM | 0.0231 mL | 0.1153 mL | 0.2307 mL | 0.4614 mL | 0.5767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rocilinostat (ACY-1215) is a selective inhibitor of HDAC6 with IC50 of 5 nM [1].
HDAC6 (histone deacetylase 6) is an enzyme which encoded by the HDAC6 gene and plays an important role in translational regulation, cell cycle progression and developmental events. It has been revealed that over-expression of HDAC6 is correlated with tumorigenesis and cell survival and HDAC6 also involves in cancer cells metastasis [2] [3].
Rocilinostat (ACY-1215) is a selective HDAC6 inhibitor. When tested with human MM cell lines (MM.1S), treatment with ACY-1215 in increasing dose for 6 hours increased acetylated α-tubulin at a concentration of 0.62μM via inhibiting HDAC6 [1]. In multiple myeloma cells, used ACY-125 as an adjuvant with carfilzomib triggered synergistic anti-MM effects, even in bortezomib-resistant cells which resulted in enhance cells apoptosis [2].
In mouse model with xenografted human MM cells, administration of ACY-125 only or with bortezomib could markedly delay tumor growth and the combination showed best efficacy [1].
It is also reported that ACY-1215 has minimal activity against HDAC4, HDAC5, HDAC7, HDAC9, HDAC11, Sirtuin1, and Sirtuin2 (IC50 > 1μM), and has slight activity against HDAC8 (IC50 = 0.1μM) [1]. When tested with mouse xenografted MM cells, combination carfilzomib with ACY-1215 showed a decreased effect on tumor volume and cells apoptosis [2].
References:
[1]. Santo, L., et al., Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood, 2012. 119(11): p. 2579-89.
[2]. Mishima, Y., et al., Ricolinostat (ACY-1215) induced inhibition of aggresome formation accelerates carfilzomib-induced multiple myeloma cell death. Br J Haematol, 2015.
[3]. Haakenson, J., et al., HDAC6-Dependent Functions in Tumor Cells: Crossroad with the MAPK Pathways. Crit Rev Oncog, 2015. 20(1-2): p. 65-81.
- (+)-Pteryxin
Catalog No.:BCN3470
CAS No.:13161-75-6
- Amyloid Beta-peptide (25-35) (human)
Catalog No.:BCC1027
CAS No.:131602-53-4
- Betulinaldehyde
Catalog No.:BCN1249
CAS No.:13159-28-9
- Calystegine A3
Catalog No.:BCN1884
CAS No.:131580-36-4
- Camelliaside B
Catalog No.:BCN3872
CAS No.:131573-90-5
- Triumbelletin
Catalog No.:BCN6779
CAS No.:131559-54-1
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- ACY-241
Catalog No.:BCC6460
CAS No.:1316215-12-9
- OPC 21268
Catalog No.:BCC7812
CAS No.:131631-89-5
- Chalepensin
Catalog No.:BCN7334
CAS No.:13164-03-9
- Turmeronol A
Catalog No.:BCN6777
CAS No.:131651-37-1
- 8-(1-Chloro-2-hydroxy-3-methylbut-3-enyl)-7-methoxycoumarin
Catalog No.:BCN7058
CAS No.:131652-35-2
- Parsonsianine
Catalog No.:BCN2110
CAS No.:131683-36-8
- (R)-(-)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8392
CAS No.:131685-53-5
- Arbidol HCl
Catalog No.:BCC3722
CAS No.:131707-23-8
- Urolignoside
Catalog No.:BCN6758
CAS No.:131723-83-6
- Flavopiridol hydrochloride
Catalog No.:BCC3925
CAS No.:131740-09-5
- DAU 5884 hydrochloride
Catalog No.:BCC7263
CAS No.:131780-48-8
- Ugaxanthone
Catalog No.:BCN6775
CAS No.:13179-11-8
Sensitive method for the determination of rocilinostat in small volume mouse plasma by LC-MS/MS and its application to a pharmacokinetic study in mice.[Pubmed:26633099]
Biomed Chromatogr. 2016 Jul;30(7):1138-1144.
A highly sensitive, specific and rapid LC-ESI-MS/MS method has been developed and validated for the quantification of rocilinostat in small volume mouse plasma (20 muL) using vorinostat as an internal standard (IS) as per regulatory guidelines. Sample preparation was accomplished through a protein precipitation procedure with acetonitrile. Chromatography was achieved on Prodigy ODS-2 column using a binary gradient using mobile phase A (0.2% formic acid in water) and B (acetonitrile) at a flow rate of 0.38 mL/min. The total chromatographic run time was 4.1 min and the elution of rocilinostat and IS occurred at ~3.2 and 2.9 min, respectively. A linear response function was established in the concentration range of 0.28-1193 ng/mL in mouse plasma. The intra- and inter-day accuracy and precisions were in the ranges of 3.12-8.93 and 6.41-11.6%, respectively. This novel method has been applied to a pharmacokinetic study in mice. Copyright (c) 2016 John Wiley & Sons, Ltd.
Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial.[Pubmed:27646843]
Lancet Oncol. 2016 Nov;17(11):1569-1578.
BACKGROUND: Histone deacetylase (HDAC) inhibitors are an important new class of therapeutics for treating multiple myeloma. Ricolinostat (ACY-1215) is the first oral selective HDAC6 inhibitor with reduced class I HDAC activity to be studied clinically. Motivated by findings from preclinical studies showing potent synergistic activity with ricolinostat and lenalidomide, our goal was to assess the safety and preliminary activity of the combination of ricolinostat with lenalidomide and dexamethasone in relapsed or refractory multiple myeloma. METHODS: In this multicentre phase 1b trial, we recruited patients aged 18 years or older with previously treated relapsed or refractory multiple myeloma from five cancer centres in the USA. Inclusion criteria included a Karnofsky Performance Status score of at least 70, measureable disease, adequate bone marrow reserve, adequate hepatic function, and a creatinine clearance of at least 50 mL per min. Exclusion criteria included previous exposure to HDAC inhibitors; previous allogeneic stem-cell transplantation; previous autologous stem-cell transplantation within 12 weeks of baseline; active systemic infection; malignancy within the last 5 years; known or suspected HIV, hepatitis B, or hepatitis C infection; a QTc Fridericia of more than 480 ms; and substantial cardiovascular, gastrointestinal, psychiatric, or other medical disorders. We gave escalating doses (from 40-240 mg once daily to 160 mg twice daily) of oral ricolinostat according to a standard 3 + 3 design according to three different regimens on days 1-21 with a conventional 28 day schedule of oral lenalidomide (from 15 mg [in one cohort] to 25 mg [in all other cohorts] once daily) and oral dexamethasone (40 mg weekly). Primary outcomes were dose-limiting toxicities, the maximum tolerated dose of ricolinostat in this combination, and the dose and schedule of ricolinostat recommended for further phase 2 investigation. Secondary outcomes were the pharmacokinetics and pharmacodynamics of ricolinostat in this combination and the preliminary anti-tumour activity of this treatment. The trial is closed to accrual and is registered at ClinicalTrials.gov, number NCT01583283. FINDINGS: Between July 12, 2012, and Aug 20, 2015, we enrolled 38 patients. We observed two dose-limiting toxicities with ricolinostat 160 mg twice daily: one (2%) grade 3 syncope and one (2%) grade 3 myalgia event in different cohorts. A maximum tolerated dose was not reached. We chose ricolinostat 160 mg once daily on days 1-21 of a 28 day cycle as the recommended dose for future phase 2 studies in combination with lenalidomide 25 mg and dexamethasone 40 mg. The most common adverse events were fatigue (grade 1-2 in 14 [37%] patients; grade 3 in seven [18%]) and diarrhoea (grade 1-2 in 15 [39%] patients; grade 3 in two [5%]). Our pharmacodynamic studies showed that at clinically relevant doses, ricolinostat selectively inhibits HDAC6 while retaining a low and tolerable level of class I HDAC inhibition. The pharmacokinetics of ricolinostat and lenalidomide were not affected by co-administration. In a preliminary assessment of antitumour activity, 21 (55% [95% CI 38-71]) of 38 patients had an overall response. INTERPRETATION: The findings from this study provide preliminary evidence that ricolinostat is a safe and well tolerated selective HDAC6 inhibitor, which might partner well with lenalidomide and dexamethasone to enhance their efficacy in relapsed or refractory multiple myeloma. FUNDING: Acetylon Pharmaceuticals.
Histone deacetylase 6 promotes growth of glioblastoma through inhibition of SMAD2 signaling.[Pubmed:26150340]
Tumour Biol. 2015 Dec;36(12):9661-5.
Histone deacetylases (HDACs) play a role in the tumorigenesis of glioblastoma multiforme (GBM), whereas the underlying mechanism has not been elucidated. Here, we reported significantly higher HDAC6 levels in GBM from the patients. GBM cell growth was significantly inhibited by ACY-1215, a specific HDAC6 inhibitor. Further analyses show that HDAC6 may promote growth of GBM cells through inhibition of SMAD2 phosphorylation to downregulate p21. Thus, our data demonstrate a previously unrecognized regulation pathway in that HDAC6 increases GBM growth through attenuating transforming growth factor beta (TGFbeta) receptor signaling.
ACY-1215 accelerates vemurafenib induced cell death of BRAF-mutant melanoma cells via induction of ER stress and inhibition of ERK activation.[Pubmed:28035401]
Oncol Rep. 2017 Feb;37(2):1270-1276.
BRAFV600E mutation is found in ~50% of melanoma patients and BRAFV600E kinase activity inhibitor, vemurafenib, has achieved a remarkable clinical response rate. However, most patients treated with vemurafenib eventually develop resistance. Overcoming primary and secondary resistance to selective BRAF inhibitors remains one of the most critically compelling challenges for these patients. HDAC6 has been shown to confer resistance to chemotherapy in several types of cancer. Few studies focused on the role of HDAC6 in vemurafenib resistance. Here we showed that overexpression of HDAC6 confers resistance to vemurafenib in BRAF-mutant A375 cells. ACY-1215, a selective HDAC6 inhibitor, inhibits the proliferation and induces the apoptosis of A375 cells. Moreover, ACY-1215 sensitizes A375 cells to vemurafenib induced cell proliferation inhibition and apoptosis induction, which occur partly through induction of endoplasmic reticulum (ER) stress and inactivation of extracellular signal-regulated kinase (ERK). Taken together, our results suggest that the inhibition of HDAC6 may be a promising strategy for the treatment of melanoma and overcoming resistance to vemurafenib.
Selectivity and Kinetic Requirements of HDAC Inhibitors as Progranulin Enhancers for Treating Frontotemporal Dementia.[Pubmed:28712747]
Cell Chem Biol. 2017 Jul 20;24(7):892-906.e5.
Frontotemporal dementia (FTD) arises from neurodegeneration in the frontal, insular, and anterior temporal lobes. Autosomal dominant causes of FTD include heterozygous mutations in the GRN gene causing haploinsufficiency of progranulin (PGRN) protein. Recently, histone deacetylase (HDAC) inhibitors have been identified as enhancers of PGRN expression, although the mechanisms through which GRN is epigenetically regulated remain poorly understood. Using a chemogenomic toolkit, including optoepigenetic probes, we show that inhibition of class I HDACs is sufficient to upregulate PGRN in human neurons, and only inhibitors with apparent fast binding to their target HDAC complexes are capable of enhancing PGRN expression. Moreover, we identify regions in the GRN promoter in which elevated H3K27 acetylation and transcription factor EB (TFEB) occupancy correlate with HDAC-inhibitor-mediated upregulation of PGRN. These findings have implications for epigenetic and cis-regulatory mechanisms controlling human GRN expression and may advance translational efforts to develop targeted therapeutics for treating PGRN-deficient FTD.


