TriumbelletinCAS# 131559-54-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131559-54-1 | SDF | Download SDF |
| PubChem ID | 14213530 | Appearance | Powder |
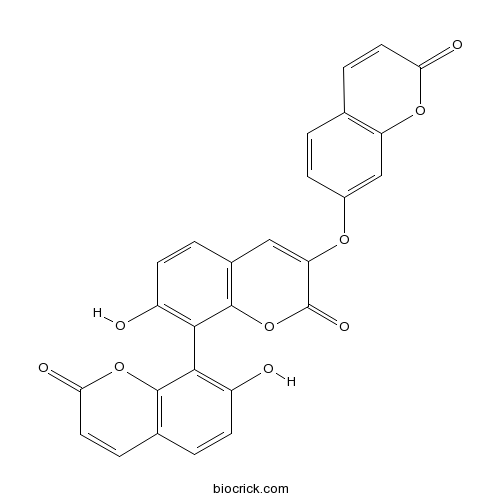
| Formula | C27H14O9 | M.Wt | 482.39 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-hydroxy-8-(7-hydroxy-2-oxochromen-8-yl)-3-(2-oxochromen-7-yl)oxychromen-2-one | ||
| SMILES | C1=CC(=CC2=C1C=CC(=O)O2)OC3=CC4=C(C(=C(C=C4)O)C5=C(C=CC6=C5OC(=O)C=C6)O)OC3=O | ||
| Standard InChIKey | GDHDMCIEKMVPIP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H14O9/c28-17-7-2-14-5-10-22(31)35-25(14)23(17)24-18(29)8-3-15-11-20(27(32)36-26(15)24)33-16-6-1-13-4-9-21(30)34-19(13)12-16/h1-12,28-29H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Triumbelletin is a natural product from Wikstroemia indica. |
| In vitro | Phenolic Constituents from Rhizome of Wikstroemia indica and Their Anti-tumor Activity[Reference: WebLink]Natural Product Research & Development, 2014 , 26 (6) :851-5.
|
| Structure Identification | J Sep Sci. 2015 Jun;38(12):2093-100.Ultra high performance liquid chromatography with electrospray ionization tandem mass spectrometry coupled with hierarchical cluster analysis to evaluate Wikstroemia indica (L.) C. A. Mey. from different geographical regions.[Pubmed: 25866087]
|

Triumbelletin Dilution Calculator

Triumbelletin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.073 mL | 10.3651 mL | 20.7301 mL | 41.4602 mL | 51.8253 mL |
| 5 mM | 0.4146 mL | 2.073 mL | 4.146 mL | 8.292 mL | 10.3651 mL |
| 10 mM | 0.2073 mL | 1.0365 mL | 2.073 mL | 4.146 mL | 5.1825 mL |
| 50 mM | 0.0415 mL | 0.2073 mL | 0.4146 mL | 0.8292 mL | 1.0365 mL |
| 100 mM | 0.0207 mL | 0.1037 mL | 0.2073 mL | 0.4146 mL | 0.5183 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- pep2-EVKI
Catalog No.:BCC5786
CAS No.:1315378-67-6
- NG25
Catalog No.:BCC1799
CAS No.:1315355-93-1
- Camelliaside B
Catalog No.:BCN3872
CAS No.:131573-90-5
- Calystegine A3
Catalog No.:BCN1884
CAS No.:131580-36-4
- Betulinaldehyde
Catalog No.:BCN1249
CAS No.:13159-28-9
- Amyloid Beta-peptide (25-35) (human)
Catalog No.:BCC1027
CAS No.:131602-53-4
- (+)-Pteryxin
Catalog No.:BCN3470
CAS No.:13161-75-6
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- ACY-241
Catalog No.:BCC6460
CAS No.:1316215-12-9
- OPC 21268
Catalog No.:BCC7812
CAS No.:131631-89-5
- Chalepensin
Catalog No.:BCN7334
CAS No.:13164-03-9
- Turmeronol A
Catalog No.:BCN6777
CAS No.:131651-37-1
- 8-(1-Chloro-2-hydroxy-3-methylbut-3-enyl)-7-methoxycoumarin
Catalog No.:BCN7058
CAS No.:131652-35-2
- Parsonsianine
Catalog No.:BCN2110
CAS No.:131683-36-8
Ultra high performance liquid chromatography with electrospray ionization tandem mass spectrometry coupled with hierarchical cluster analysis to evaluate Wikstroemia indica (L.) C. A. Mey. from different geographical regions.[Pubmed:25866087]
J Sep Sci. 2015 Jun;38(12):2093-100.
A sensitive, rapid and simple ultra high performance liquid chromatography with electrospray ionization tandem mass spectrometry method was developed to determine seven constituents (umbelliferone, apigenin, Triumbelletin, daphnoretin, arctigenin, genkwanin and emodin) in Wikstroemia indica (L.) C. A. Mey. The chromatographic analysis was performed on an ACQUITY UPLC(R) BEH C18 column (2.1 x 50 mm, 1.7 mum) by gradient elution with the mobile phase of 0.05% formic acid aqueous solution (A) and acetonitrile (B). Multiple reaction monitoring mode with positive and negative electrospray ionization interface was carried out to detect the components. This method was validated in terms of specificity, linearity, accuracy, precision and stability. Excellent linear behavior was observed over the certain concentration ranges with the correlation coefficient values higher than 0.999. The intraday and innerday precisions were within 2.0%. The recoveries of seven analytes were 99.4-101.1% with relative standard deviation less than 1.2%. The 18 Wikstroemia indica samples from different origins were classified by hierarchical clustering analysis according to the contents of seven components. The results demonstrated that the developed method could successfully be used to quantify simultaneously of seven components in Wikstroemia indica and could be a helpful tool for the detection and confirmation of the quality of traditional Chinese medicines.


