NG25CAS# 1315355-93-1 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1315355-93-1 | SDF | Download SDF |
| PubChem ID | 53340664 | Appearance | Powder |
| Formula | C29H30F3N5O2 | M.Wt | 537.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (186.02 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
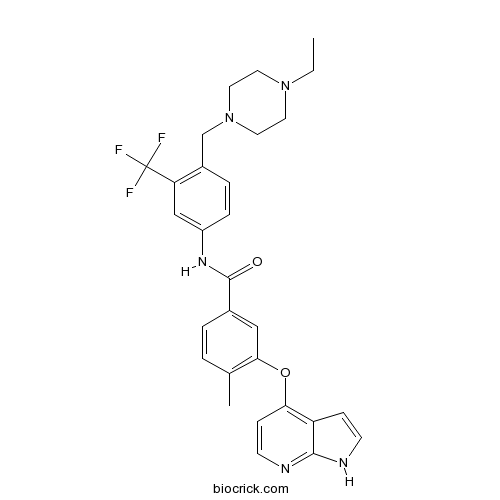
| Chemical Name | N-[4-[(4-ethylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]-4-methyl-3-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)benzamide | ||
| SMILES | CCN1CCN(CC1)CC2=C(C=C(C=C2)NC(=O)C3=CC(=C(C=C3)C)OC4=C5C=CNC5=NC=C4)C(F)(F)F | ||
| Standard InChIKey | SMPGEBOIKULBCT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H30F3N5O2/c1-3-36-12-14-37(15-13-36)18-21-6-7-22(17-24(21)29(30,31)32)35-28(38)20-5-4-19(2)26(16-20)39-25-9-11-34-27-23(25)8-10-33-27/h4-11,16-17H,3,12-15,18H2,1-2H3,(H,33,34)(H,35,38) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TGF-β-activated kinase (TAK1) inhibitor (IC50 = 149 nM). Also inhibits LYN, MAP4K2 and Abl kinase activity (IC50 values are 12.9, 21.7 and 75.2 nM, respectively). Suppresses CpG A and CpG B-stimulated secretion of IFN-α and TLR7/8 agonist CL097-stimulated secretion of IFN-β in Gen 2.2 leukemia cells in vitro. |

NG25 Dilution Calculator

NG25 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8602 mL | 9.3009 mL | 18.6019 mL | 37.2038 mL | 46.5047 mL |
| 5 mM | 0.372 mL | 1.8602 mL | 3.7204 mL | 7.4408 mL | 9.3009 mL |
| 10 mM | 0.186 mL | 0.9301 mL | 1.8602 mL | 3.7204 mL | 4.6505 mL |
| 50 mM | 0.0372 mL | 0.186 mL | 0.372 mL | 0.7441 mL | 0.9301 mL |
| 100 mM | 0.0186 mL | 0.093 mL | 0.186 mL | 0.372 mL | 0.465 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NG25, a TAK1 inhibitor, inhibited the activation of IKK by TLR7 and TLR9 agonists and prevented the secretion of type 1 IFNs induced by these ligands in Gen2.2 cells.
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- Amyloid Beta-Peptide (1-40) (human)
Catalog No.:BCC1045
CAS No.:131438-79-4
- Cercosporamide
Catalog No.:BCC2438
CAS No.:131436-22-1
- UNC669
Catalog No.:BCC3997
CAS No.:1314241-44-5
- TC-N 22A
Catalog No.:BCC6150
CAS No.:1314140-00-5
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Boc-Ile-OH.1/2H2O
Catalog No.:BCC3406
CAS No.:13139-16-7
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- Boc-Trp-OH
Catalog No.:BCC3455
CAS No.:13139-14-5
- pep2-EVKI
Catalog No.:BCC5786
CAS No.:1315378-67-6
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- Triumbelletin
Catalog No.:BCN6779
CAS No.:131559-54-1
TAK1 inhibitor NG25 enhances doxorubicin-mediated apoptosis in breast cancer cells.[Pubmed:27599572]
Sci Rep. 2016 Sep 7;6:32737.
Doxorubicin (Dox, Adriamycin) has been widely used in breast cancer treatment. But its severe cardio-toxic side effects limited the clinical use. Dox treatment can induce DNA damage and other accompanying effects in cancer cells, and subsequently activates nuclear factor kappaB (NF-kappaB) pathway which has a strong pro-survival role in different types of malignancy. We hypothesize that blocking NF-kappaB pathway may sensitize breast cancer cells to Dox chemotherapy. TGFbeta-activated kinase-1 (TAK1) is a key intracellular molecule participating in genotoxic stresses-induced NF-kappaB activation. Targeting TAK1 as a strategy to enhance cancer treatment efficacy has been studied in several malignancies. We showed that NG25, a synthesized TAK1 inhibitor, greatly enhanced Dox treatment efficacy in a panel of breast cancer cell lines. In this pre-clinical study, we found that NG25 partially blocked Dox-induced p38 phosphorylation and IkappaBalpha degradation and enhanced Dox-induced cytotoxic effects and apoptosis in all breast cancer cell lines tested. Taken together, we provided clear evidence that NG25 sensitizes the breast cancer cells to Dox treatment in vitro. This combination may be an effective and feasible therapeutic option maximizing Dox efficacy and meanwhile minimizing Dox side effects in treating breast cancer.
Discovery of type II inhibitors of TGFbeta-activated kinase 1 (TAK1) and mitogen-activated protein kinase kinase kinase kinase 2 (MAP4K2).[Pubmed:25075558]
J Med Chem. 2015 Jan 8;58(1):183-96.
We developed a pharmacophore model for type II inhibitors that was used to guide the construction of a library of kinase inhibitors. Kinome-wide selectivity profiling of the library resulted in the identification of a series of 4-substituted 1H-pyrrolo[2,3-b]pyridines that exhibited potent inhibitory activity against two mitogen-activated protein kinases (MAPKs), TAK1 (MAP3K7) and MAP4K2, as well as pharmacologically well interrogated kinases such as p38alpha (MAPK14) and ABL. Further investigation of the structure-activity relationship (SAR) resulted in the identification of potent dual TAK1 and MAP4K2 inhibitors such as 1 (NG25) and 2 as well as MAP4K2 selective inhibitors such as 16 and 17. Some of these inhibitors possess good pharmacokinetic properties that will enable their use in pharmacological studies in vivo. A 2.4 A cocrystal structure of TAK1 in complex with 1 confirms that the activation loop of TAK1 assumes the DFG-out conformation characteristic of type II inhibitors.
The IkappaB kinase family phosphorylates the Parkinson's disease kinase LRRK2 at Ser935 and Ser910 during Toll-like receptor signaling.[Pubmed:22723946]
PLoS One. 2012;7(6):e39132.
Mutations in leucine-rich repeat kinase 2 (LRRK2) are strongly associated with late-onset autosomal dominant Parkinson's disease. LRRK2 is highly expressed in immune cells and recent work points towards a link between LRRK2 and innate immunity. Here we demonstrate that stimulation of the Toll-Like Receptor (TLR) pathway by MyD88-dependent agonists in bone marrow-derived macrophages (BMDMs) or RAW264.7 macrophages induces marked phosphorylation of LRRK2 at Ser910 and Ser935, the phosphorylation sites that regulate the binding of 14-3-3 to LRRK2. Phosphorylation of these residues is prevented by knock-out of MyD88 in BMDMs, but not the alternative TLR adaptor protein TRIF. Utilising both pharmacological inhibitors, including a new TAK1 inhibitor, NG25, and genetic models, we provide evidence that both the canonical (IKKalpha and IKKbeta) and IKK-related (IKKepsilon and TBK1) kinases mediate TLR agonist induced phosphorylation of LRRK2 in vivo. Moreover, all four IKK members directly phosphorylate LRRK2 at Ser910 and Ser935 in vitro. Consistent with previous work describing Ser910 and Ser935 as pharmacodynamic biomarkers of LRRK2 activity, we find that the TLR independent basal phosphorylation of LRRK2 at Ser910 and Ser935 is abolished following treatment of macrophages with LRRK2 kinase inhibitors. However, the increased phosphorylation of Ser910 and Ser935 induced by activation of the MyD88 pathway is insensitive to LRRK2 kinase inhibitors. Finally, employing LRRK2-deficient BMDMs, we present data indicating that LRRK2 does not play a major role in regulating the secretion of inflammatory cytokines induced by activation of the MyD88 pathway. Our findings provide the first direct link between LRRK2 and the IKKs that mediate many immune responses. Further work is required to uncover the physiological roles that phosphorylation of LRRK2 by IKKs play in controlling macrophage biology and to determine how phosphorylation of LRRK2 by IKKs impacts upon the use of Ser910 and Ser935 as pharmacodynamic biomarkers.
Essential role for IKKbeta in production of type 1 interferons by plasmacytoid dendritic cells.[Pubmed:22511786]
J Biol Chem. 2012 Jun 1;287(23):19216-28.
Plasmacytoid dendritic cells (pDCs) are characterized by their ability to produce high levels of type 1 interferons in response to ligands that activate TLR7 and TLR9, but the signaling pathways required for IFN production are incompletely understood. Here we exploit the human pDC cell line Gen2.2 and improved pharmacological inhibitors of protein kinases to address this issue. We demonstrate that ligands that activate TLR7 and TLR9 require the TAK1-IKKbeta signaling pathway to induce the production of IFNbeta via a pathway that is independent of the degradation of IkappaBalpha. We also show that IKKbeta activity, as well as the subsequent IFNbeta-stimulated activation of the JAK-STAT1/2 signaling pathway, are essential for the production of IFNalpha by TLR9 ligands. We further show that TLR7 ligands CL097 and R848 fail to produce significant amounts of IFNalpha because the activation of IKKbeta is not sustained for a sufficient length of time. The TLR7/9-stimulated production of type 1 IFNs is inhibited by much lower concentrations of IKKbeta inhibitors than those needed to suppress the production of NFkappaB-dependent proinflammatory cytokines, such as IL-6, suggesting that drugs that inhibit IKKbeta may have a potential for the treatment of forms of lupus that are driven by self-RNA and self-DNA-induced activation of TLR7 and TLR9, respectively.


