Amyloid Beta-Peptide (1-40) (human)Amyloid precursor protein CAS# 131438-79-4 |
- Amyloid Beta-Peptide (12-28) (human)
Catalog No.:BCC1044
CAS No.:107015-83-8
- Amyloid Beta-peptide (25-35) (human)
Catalog No.:BCC1027
CAS No.:131602-53-4
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131438-79-4 | SDF | Download SDF |
| PubChem ID | 71581487 | Appearance | Powder |
| Formula | C194H295N53O58S | M.Wt | 4329.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
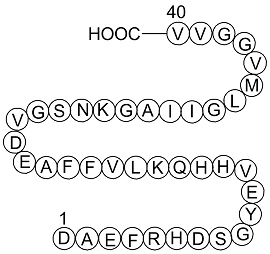
| Sequence | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGA | ||
| SMILES | CCC(C)C(C(=O)NC(C(C)CC)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(CCSC)C(=O)NC(C(C)C)C(=O)NCC(=O)NCC(=O)NC(C(C)C)C(=O)NC(C(C)C)C(=O)O)NC(=O)C(C)NC(=O)CNC(=O)C(CCCCN)NC(=O)C(CC(=O)N)NC(=O)C(CO)NC(=O)CNC(=O)C(C(C)C)NC(=O)C(CC(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(C)NC(=O)C(CC1=CC=CC=C1)NC(=O)C(CC2=CC=CC=C2)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CCCCN)NC(=O)C(CCC(=O)N)NC(=O)C(CC3=CNC=N3)NC(=O)C(CC4=CNC=N4)NC(=O)C(C(C)C)NC(=O)C(CCC(=O)O)NC(=O)C(CC5=CC=C(C=C5)O)NC(=O)CNC(=O)C(CO)NC(=O)C(CC(=O)O)NC(=O)C(CC6=CNC=N6)NC(=O)C(CCCNC(=N)N)NC(=O)C(CC7=CC=CC=C7)NC(=O)C(CCC(=O)O)NC(=O)C(C)NC(=O)C(CC(=O)O)N | ||
| Standard InChIKey | FEWOUVRMGWFWIH-SRXWUVNJSA-N | ||
| Standard InChI | InChI=1S/C194H295N53O58S/c1-25-102(19)158(188(299)211-87-143(256)218-124(67-94(3)4)174(285)228-123(62-66-306-24)172(283)241-152(96(7)8)186(297)209-83-140(253)206-84-145(258)240-154(98(11)12)191(302)245-157(101(17)18)193(304)305)247-192(303)159(103(20)26-2)246-162(273)104(21)215-141(254)85-207-164(275)116(47-36-38-63-195)223-181(292)133(77-139(199)252)234-185(296)137(90-249)220-144(257)88-210-187(298)153(97(9)10)242-184(295)135(79-151(269)270)235-170(281)121(56-60-147(261)262)222-161(272)106(23)217-173(284)127(69-107-41-30-27-31-42-107)231-177(288)129(71-109-45-34-29-35-46-109)237-189(300)156(100(15)16)244-183(294)125(68-95(5)6)229-166(277)117(48-37-39-64-196)224-168(279)119(54-58-138(198)251)226-178(289)130(73-111-80-202-91-212-111)233-180(291)132(75-113-82-204-93-214-113)238-190(301)155(99(13)14)243-171(282)122(57-61-148(263)264)227-175(286)126(72-110-50-52-114(250)53-51-110)219-142(255)86-208-165(276)136(89-248)239-182(293)134(78-150(267)268)236-179(290)131(74-112-81-203-92-213-112)232-167(278)118(49-40-65-205-194(200)201)225-176(287)128(70-108-43-32-28-33-44-108)230-169(280)120(55-59-146(259)260)221-160(271)105(22)216-163(274)115(197)76-149(265)266/h27-35,41-46,50-53,80-82,91-106,115-137,152-159,248-250H,25-26,36-40,47-49,54-79,83-90,195-197H2,1-24H3,(H2,198,251)(H2,199,252)(H,202,212)(H,203,213)(H,204,214)(H,206,253)(H,207,275)(H,208,276)(H,209,297)(H,210,298)(H,211,299)(H,215,254)(H,216,274)(H,217,284)(H,218,256)(H,219,255)(H,220,257)(H,221,271)(H,222,272)(H,223,292)(H,224,279)(H,225,287)(H,226,289)(H,227,286)(H,228,285)(H,229,277)(H,230,280)(H,231,288)(H,232,278)(H,233,291)(H,234,296)(H,235,281)(H,236,290)(H,237,300)(H,238,301)(H,239,293)(H,240,258)(H,241,283)(H,242,295)(H,243,282)(H,244,294)(H,245,302)(H,246,273)(H,247,303)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H,267,268)(H,269,270)(H,304,305)(H4,200,201,205)/t102?,103?,104-,105+,106+,115?,116-,117+,118+,119+,120+,121-,122+,123-,124-,125+,126+,127+,128+,129+,130+,131+,132+,133-,134+,135-,136+,137-,152-,153-,154-,155+,156+,157-,158-,159-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide found in plaques in the brains of patients with Alzheimer's disease. Shown to have both neurotrophic and neurotoxic effects. |

Amyloid Beta-Peptide (1-40) (human) Dilution Calculator

Amyloid Beta-Peptide (1-40) (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Amyloid β-Peptide (1-40) (human), (C194H295N53O58S1), a peptide with the sequence H2N-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA-OH, MW= 4329.8. Amyloid beta (Aβ or Abeta) is a peptide of 36–43 amino acids that is processed from the Amyloid precursor protein. While best known as a component of amyloid plaques in association with Alzheimer's disease, evidence has been found that Aβ is a highly multifunctional peptide with significant non-pathological activity(1). Aβ is the main component of deposits found in the brains of patients with Alzheimer's disease. Brain Aβ is elevated in patients with sporadic Alzheimer’s disease. Aβ is the main constituent of brain parenchymal and vascular amyloid, it contributes to cerebrovascular lesions and is neurotoxic(2). Aβ protein is generated by successive action of the β and γ secretases. The γ secretase, which produces the C-terminal end of the Aβ peptide, cleaves within the transmembrane region of APP and can generate a number of isoforms of 36-43 amino acid residues in length. The most common isoforms are Aβ40 and Aβ42; the longer form is typically produced by cleavage that occurs in the endoplasmic reticulum, while the shorter form is produced by cleavage in the trans-Golgi network(3).
Figure1 structure of Amyloid β-Peptide (1-40) (human)
Ref:
1. Lahiri DK, Maloney B (September 2010). "Beyond the signaling effect role of amyloid–β42 on the processing of AβPP, and its clinical implications". Exp. Neurol. 225 (1): 51–4.
2. Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M (September 1998). "Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau". Nat. Neurosci. 1 (5): 355–8.
3. Hartmann T, Bieger SC, Brühl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K (September 1997). "Distinct sites of intracellular production for Alzheimer's disease A beta40/42 amyloid peptides". Nat. Med. 3 (9): 1016–20.
- Cercosporamide
Catalog No.:BCC2438
CAS No.:131436-22-1
- UNC669
Catalog No.:BCC3997
CAS No.:1314241-44-5
- TC-N 22A
Catalog No.:BCC6150
CAS No.:1314140-00-5
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Boc-Ile-OH.1/2H2O
Catalog No.:BCC3406
CAS No.:13139-16-7
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- Boc-Trp-OH
Catalog No.:BCC3455
CAS No.:13139-14-5
- Teijin compound 1
Catalog No.:BCC6057
CAS No.:1313730-14-1
- Periplocin
Catalog No.:BCN2655
CAS No.:13137-64-9
- erythro-Guaiacylglycerol beta-threo-syringylglycerol ether
Catalog No.:BCN7333
CAS No.:1313434-74-0
- NVP-CGM097
Catalog No.:BCC5395
CAS No.:1313363-54-0
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- NG25
Catalog No.:BCC1799
CAS No.:1315355-93-1
- pep2-EVKI
Catalog No.:BCC5786
CAS No.:1315378-67-6
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
Contributions of degradation and brain-to-blood elimination across the blood-brain barrier to cerebral clearance of human amyloid-beta peptide(1-40) in mouse brain.[Pubmed:23963369]
J Cereb Blood Flow Metab. 2013 Nov;33(11):1770-7.
The purpose of the present study was to estimate the relative contributions of degradation and brain-to-blood elimination processes to the clearance of microinjected human amyloid-beta peptide(1-40) (hAbeta(1-40)) from mouse cerebral cortex, using a solid-phase extraction method together with a newly developed ultraperformance liquid chromatography/tandem mass spectrometry (UPLC/MS/MS) quantitation method for intact hAbeta(1-40). The clearance rate constant of hAbeta(1-40) in mouse cerebral cortex was determined to be 3.21 x 10(-2)/min under conditions where the saturable brain-to-blood elimination process across the blood-brain barrier (BBB) was expected to be saturated. Thus, this clearance rate constant should mainly reflect degradation. The [(125)I]hAbeta(1-40) elimination rate across the BBB under nonsaturating conditions was determined to be 1.48 x 10(-2)/min. Inhibition studies suggested that processes sensitive to insulin and phosphoramidon, which inhibit neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme, are involved not only in degradation, but also in elimination of hAbeta(1-40). In conclusion, our results suggest a dominant contribution of degradation to cerebral hAbeta(1-40) clearance, and also indicate that a sequential process of degradation and elimination of degradation products is involved in cerebral hAbeta(1-40) clearance.
1alpha,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-beta peptide(1-40) from mouse brain across the blood-brain barrier.[Pubmed:21740543]
Fluids Barriers CNS. 2011 Jul 8;8:20.
BACKGROUND: Cerebrovascular dysfunction has been considered to cause impairment of cerebral amyloid-beta peptide (Abeta) clearance across the blood-brain barrier (BBB). Further, low levels of vitamin D are associated with increased risk of Alzheimer's disease, as well as vascular dysfunction. The purpose of the present study was to investigate the effect of 1alpha,25-dihydroxyvitamin D3 (1,25(OH)2D3), an active form of vitamin D, on cerebral Abeta clearance from mouse brain. METHODS: The elimination of [125I]hAbeta(1-40) from mouse brain was examined by using the Brain Efflux Index method to determine the remaining amount of [125I]hAbeta(1-40) radioactivity after injection into the cerebral cortex. [125I]hAbeta(1-40) internalization was analyzed using conditionally immortalized mouse brain capillary endothelial cells (TM-BBB4). RESULTS: Twenty-four hours after intraperitoneal injection of 1,25(OH)2D3 (1 mug/mouse), [125I]hAbeta(1-40) elimination from mouse brain was increased 1.3-fold, and the level of endogenous Abeta(1-40) in mouse brain was reduced. These effects were observed at 24 h after i.p. injection of 1,25(OH)2D3, while no significant effect was observed at 48 or 72 h. Vitamin D receptor (VDR) mRNA was detected in mouse brain capillaries, suggesting that 1,25(OH)2D3 has a VDR-mediated genomic action. Furthermore, forskolin, which activates mitogen-activated protein kinase kinase (MEK), enhanced [125I]hAbeta(1-40) elimination from mouse brain. Forskolin also enhanced [125I]hAbeta(1-40) internalization in TM-BBB4 cells, and this enhancement was inhibited by a MEK inhibitor, suggesting involvement of non-genomic action. CONCLUSIONS: The active form of vitamin D, 1,25(OH)2D3, appears to enhance brain-to-blood Abeta(1-40) efflux transport at the BBB through both genomic and non-genomic actions. Compounds activating these pathways may be candidate agents for modulating Abeta(1-40) elimination at the BBB.
ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-beta peptide (1-40) at the blood-brain barrier.[Pubmed:18201804]
Neurochem Int. 2008 May;52(6):956-61.
ATP-binding cassette transporter A1 (ABCA1) mediates apolipoprotein-dependent cholesterol release from cellular membranes. Recent studies using ABCA1 knockout mice have demonstrated that ABCA1 affects amyloid-beta peptide (A beta) levels in the brain and the production of senile plaque. Cerebral A beta(1-40) was eliminated from the brain to the circulating blood via the blood-brain barrier (BBB), which expresses ABCA1. Therefore, in the present study, we examined whether ABCA1 affects the brain-to-blood efflux transport of human A beta(1-40)(hA beta(1-40)) at the BBB. The apparent uptake of [125I]hA beta(1-40) into ABCA1-expressing HEK293 cells was not significantly different from that into parental HEK293 cells. In addition, the apparent uptake was not significantly affected even in the presence of apolipoprotein A-I as a cholesterol release acceptor. Moreover, [125I]hA beta(1-40) elimination from mouse brain across the BBB was not significantly different between ABCA1-deficient and wild-type mice 60 min after its administration into the cerebrum. These results suggest that ABCA1 does not directly transport hA beta(1-40) and a deficiency of ABCA1 does not attenuate the brain-to-blood efflux transport of hA beta(1-40) across the BBB.
Cerebral clearance of human amyloid-beta peptide (1-40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes.[Pubmed:17908238]
J Neurochem. 2007 Dec;103(6):2482-90.
Soluble amyloid-beta peptide (Abeta) exists in the form of monomers and oligomers, and as complexes with Abeta-binding molecules, such as low-density lipoprotein receptor-related protein-1 (LRP-1) ligands. The present study investigated the effect of self-aggregation and LRP-1 ligands on the elimination of human Abeta(1-40) [hAbeta(1-40)] from the rat brain across the blood-brain barrier. Incubation of [(125)I]hAbeta(1-40) monomer resulted in time-dependent and temperature-dependent dimer formation, and the apparent elimination rate of [(125)I]hAbeta(1-40) dimer was significantly decreased by 92.7% compared with that of [(125)I]hAbeta(1-40) monomer. Pre-incubation with LRP-1 ligands, such as activated alpha2-macroglobulin (alpha2M), apolipoprotein E2 (apoE2), apoE3, apoE4, and lactoferrin, reduced the elimination of [(125)I]hAbeta(1-40). By contrast, pre-administration of the same concentration of these molecules in the rat brain did not significantly inhibit [(125)I]hAbeta(1-40) monomer elimination. Purified [(125)I]hAbeta(1-40)/activated alpha2M complex and [(125)I]activated alpha2M were not significantly eliminated from the rat brain up to 60 min. MEF-1 cells, which have LRP-1-mediated endocytosis, exhibited uptake of [(125)I]activated alpha2M, and enhancement of [(125)I]hAbeta(1-40) uptake upon pre-incubation with apoE, suggesting that [(125)I]activated alpha2M and [(125)I]hAbeta(1-40)/apoE complex function as LRP-1 ligands. These findings indicate that dimerization and LRP-1-ligand complex formation prevent the elimination of hAbeta(1-40) from the brain across the blood-brain barrier.
Beta-amyloid(1-40)-induced neurodegeneration in the rat hippocampal neurons of the CA1 subfield.[Pubmed:9600591]
Acta Neuropathol. 1998 May;95(5):455-65.
Small volumes of solutions injected into the hippocampus produce dramatic degeneration in dentate gyrus neurons, but not in neurons of the CA1 subfield. The aim of the present study was to ascertain whether solutions with different fragments of the beta-amyloid protein (Abeta) could produce further degeneration in areas beyond the dentate gyrus. It was found that 5 days after injection of an aqueous solution containing the Abeta 1-40 fragment into the hippocampus, long stretches of the CA1 subfield were either deprived of neurons or most of the neurons were degenerating. By contrast, in animals with deposits containing Abeta 1-28, Abeta 1-42 or water, neuronal degeneration or depletion only occurred in a reduced area around the place where the implant needle penetrated the CA1 subfield. In animals injected with Abeta 1-40, many profiles in the CA1 subfield and dentate gyrus were undergoing apoptosis, as seen using preparations processed by routine histology or the TUNEL technique for detection of fragmented DNA. In addition, there was higher infiltration by ED1-positive, activated microglia-macrophagic cells in Abeta 1-42 deposits than in deposits of Abeta 1-40. The present results suggest that the intrahippocampal injection of toxic Abeta fragments produces neuronal degeneration in the rat CA1 subfield when using the appropriate protocol, and, thus, can provide an in vivo model to investigate the neurotoxic effects of Abeta and for the evaluation of drugs with potential anti-neurodegenerative activity.
Beta-amyloid(1-40) effects on behavior and memory.[Pubmed:7552329]
Brain Res. 1995 Jun 5;682(1-2):69-74.
Beta amyloid 1-40 is a primary protein in plaques found in the brains of patients with Alzheimer's disease. There is evidence that unaggregated soluble beta-amyloid may be neurotoxic and may have behavioral effects on some types of memory. In the current study, the 1-40 fragment of beta-amyloid protein (beta A4), or vehicle, was bilaterally injected into the rostral hippocampus of rats performing under stable food-maintained schedules of reinforcement or under a delayed conditional discrimination procedure. Under the first procedure, rats were trained to stability under a multiple fixed interval 15 s, fixed ratio 30 reinforcement schedule. This reinforcement schedule has proven sensitive to low-dose drug effects. Acute bilateral hippocampal beta A4 (1.0, 2.0 and 3.0 microliters of 10(-3) M) administration did not significantly alter responding, compared to vehicle, under either reinforcement condition. Following the acute single-injection regimen, rats were administered chronic daily beta A4 (1 microliter of 10(-3) M), bilaterally, for 15 days. No significant changes in lever-pressing performance were observed during the chronic injection regimen, but performance declined significantly 30 days after termination of the chronic daily regimen. Histological examination revealed three of six rats showed positive reactions under Thioflavin S staining in and around the area of cannulae termination. The second assessment employed a delayed conditional discrimination procedure to evaluate the effects of intrahippocampal injections of beta A4 on short-term working memory. This conditional discrimination procedure assesses appropriate responding, dependent on a previously presented stimulus, after delays of various lengths have been imposed between the stimulus and the response opportunity.(ABSTRACT TRUNCATED AT 250 WORDS)
beta-Amyloid protein induces platelet aggregation and supports platelet adhesion.[Pubmed:7811271]
Biochem Biophys Res Commun. 1994 Dec 30;205(3):1829-35.
The amyloid precursor protein (APP) is found in many cells including neurons, endothelial cells and blood platelets. Beta-amyloid protein (beta AP) is derived from APP and is deposited in brain and in cerebral microvasculature of individuals with Alzheimer's disease. In this study we demonstrate that beta AP interacts with human blood platelets. We found that human beta AP peptide (1-40) fibrils aggregate platelets and support their adhesion, and these interactions are mediated through platelet membrane integrin receptors.
Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides.[Pubmed:2218531]
Science. 1990 Oct 12;250(4978):279-82.
The amyloid beta protein is deposited in the brains of patients with Alzheimer's disease but its pathogenic role is unknown. In culture, the amyloid beta protein was neurotrophic to undifferentiated hippocampal neurons at low concentrations and neurotoxic to mature neurons at higher concentrations. In differentiated neurons, amyloid beta protein caused dendritic and axonal retraction followed by neuronal death. A portion of the amyloid beta protein (amino acids 25 to 35) mediated both the trophic and toxic effects and was homologous to the tachykinin neuropeptide family. The effects of the amyloid beta protein were mimicked by tachykinin antagonists and completely reversed by specific tachykinin agonists. Thus, the amyloid beta protein could function as a neurotrophic factor for differentiating neurons, but at high concentrations in mature neurons, as in Alzheimer's disease, could cause neuronal degeneration.


