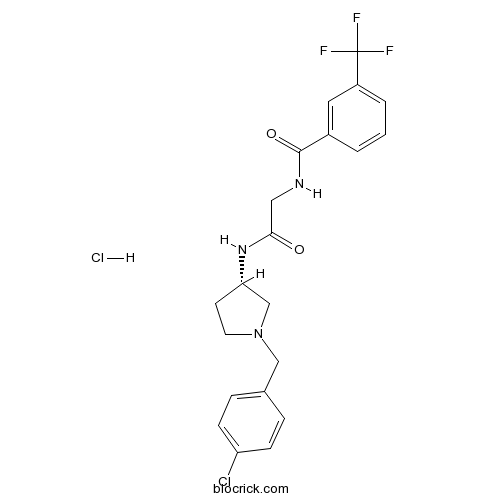
Teijin compound 1Potent CCR2b antagonist CAS# 1313730-14-1 |
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- Daclatasvir (BMS-790052)
Catalog No.:BCC2533
CAS No.:1214735-16-6
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- PSI-6130
Catalog No.:BCC1870
CAS No.:817204-33-4
- PSI-6206
Catalog No.:BCC3609
CAS No.:863329-66-2
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1313730-14-1 | SDF | Download SDF |
| PubChem ID | 124080970 | Appearance | Powder |
| Formula | C21H22Cl2F3N3O2 | M.Wt | 476.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water and to 100 mM in DMSO | ||
| Chemical Name | N-[2-[[(3S)-1-[(4-chlorophenyl)methyl]pyrrolidin-3-yl]amino]-2-oxoethyl]-3-(trifluoromethyl)benzamide;hydrochloride | ||
| SMILES | C1CN(CC1NC(=O)CNC(=O)C2=CC(=CC=C2)C(F)(F)F)CC3=CC=C(C=C3)Cl.Cl | ||
| Standard InChIKey | PGUQBBISBKGQDC-FERBBOLQSA-N | ||
| Standard InChI | InChI=1S/C21H21ClF3N3O2.ClH/c22-17-6-4-14(5-7-17)12-28-9-8-18(13-28)27-19(29)11-26-20(30)15-2-1-3-16(10-15)21(23,24)25;/h1-7,10,18H,8-9,11-13H2,(H,26,30)(H,27,29);1H/t18-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent chemokine CCR2b receptor antagonist (IC50 = 180 nM). Potently inhibits cell chemotaxis induced by MCP-1 (EC50 = 24 nM). |

Teijin compound 1 Dilution Calculator

Teijin compound 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0994 mL | 10.4971 mL | 20.9943 mL | 41.9886 mL | 52.4857 mL |
| 5 mM | 0.4199 mL | 2.0994 mL | 4.1989 mL | 8.3977 mL | 10.4971 mL |
| 10 mM | 0.2099 mL | 1.0497 mL | 2.0994 mL | 4.1989 mL | 5.2486 mL |
| 50 mM | 0.042 mL | 0.2099 mL | 0.4199 mL | 0.8398 mL | 1.0497 mL |
| 100 mM | 0.021 mL | 0.105 mL | 0.2099 mL | 0.4199 mL | 0.5249 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Periplocin
Catalog No.:BCN2655
CAS No.:13137-64-9
- erythro-Guaiacylglycerol beta-threo-syringylglycerol ether
Catalog No.:BCN7333
CAS No.:1313434-74-0
- NVP-CGM097
Catalog No.:BCC5395
CAS No.:1313363-54-0
- Nystose
Catalog No.:BCN5397
CAS No.:13133-07-8
- threo Ifenprodil hemitartrate
Catalog No.:BCC7508
CAS No.:1312991-83-5
- Ro 8-4304 hydrochloride
Catalog No.:BCC7655
CAS No.:1312991-77-7
- Ro 25-6981 Maleate
Catalog No.:BCC4159
CAS No.:1312991-76-6
- Scutebarbatine Z
Catalog No.:BCN6991
CAS No.:1312716-28-1
- Scutebarbatine Y
Catalog No.:BCN6994
CAS No.:1312716-27-0
- Scutebarbatine X
Catalog No.:BCN6997
CAS No.:1312716-26-9
- Scutebarbatine W
Catalog No.:BCN7011
CAS No.:1312716-25-8
- Antibiotic PF 1018
Catalog No.:BCN2149
CAS No.:131256-42-3
- Boc-Trp-OH
Catalog No.:BCC3455
CAS No.:13139-14-5
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- Boc-Ile-OH.1/2H2O
Catalog No.:BCC3406
CAS No.:13139-16-7
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
- TC-N 22A
Catalog No.:BCC6150
CAS No.:1314140-00-5
- UNC669
Catalog No.:BCC3997
CAS No.:1314241-44-5
- Cercosporamide
Catalog No.:BCC2438
CAS No.:131436-22-1
- Amyloid Beta-Peptide (1-40) (human)
Catalog No.:BCC1045
CAS No.:131438-79-4
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes.[Pubmed:25438248]
Eur J Pharm Biopharm. 2015 Jan;89:18-29.
Chemokines are critically involved in the development of chronic inflammatory-associated diseases such as atherosclerosis. We hypothesized that targeted delivery of compounds to the surface of activated endothelial cells (EC) interferes with chemokine/receptor interaction and thereby efficiently blocks inflammation. We developed PEGylated target-sensitive liposomes (TSL) encapsulating a CCR2 antagonist (Teijin compound 1) coupled with a specific peptide recognized by endothelial VCAM-1 (Vp-TSL-Tj). TSL were characterized for size (by dynamic light scattering), the amount of peptide coupled at the liposomal surface and Teijin release (by HPLC). We report that Vp-TSL-Tj binds specifically to activated EC in vitro and in situ, release the entrapped Teijin and prevent the transmigration of monocytes through activated EC. This is the first evidence that nanocarriers which transport and release chemokine inhibitors at specific pathological sites can reduce chemokine-dependent inflammatory processes.
Elucidation of binding sites of dual antagonists in the human chemokine receptors CCR2 and CCR5.[Pubmed:19297521]
Mol Pharmacol. 2009 Jun;75(6):1325-36.
Design of dual antagonists for the chemokine receptors CCR2 and CCR5 will be greatly facilitated by knowledge of the structural differences of their binding sites. Thus, we computationally predicted the binding site of the dual CCR2/CCR5 antagonist N-dimethyl-N-[4-[[[2-(4-methylphenyl)-6,7-dihydro-5H-benzohepten-8-yl] carbonyl]amino]benzyl]tetrahydro-2H-pyran-4-aminium (TAK-779), and a CCR2-specific antagonist N-(carbamoylmethyl)-3-trifluoromethyl benzamido-parachlorobenzyl 3-aminopyrrolidine (Teijin compound 1) in an ensemble of predicted structures of human CCR2 and CCR5. Based on our predictions of the protein-ligand interactions, we examined the activity of the antagonists for cells expressing thirteen mutants of CCR2 and five mutants of CCR5. The results show that residues Trp98(2.60) and Thr292(7.40) contribute significantly to the efficacy of both TAK-779 and Teijin compound 1, whereas His121(3.33) and Ile263(6.55) contribute significantly only to the antagonistic effect of Teijin compound 1 at CCR2. Mutation of residues Trp86(2.60) and Tyr108(3.32) adversely affected the efficacy of TAK-779 in antagonizing CCR5-mediated chemotaxis. Y49A(1.39) and E291A(7.39) mutants of CCR2 showed a complete loss of CCL2 binding and chemotaxis, despite robust cell surface expression, suggesting that these residues are critical in maintaining the correct receptor architecture. Modeling studies support the hypothesis that the residues Tyr49(1.39), Trp98(2.60), Tyr120(3.32), and Glu291(7.39) of CCR2 form a tight network of aromatic cluster and polar contacts between transmembrane helices 1, 2, 3, and 7.


