UNC669L3MBTL antagonist,potent and selective CAS# 1314241-44-5 |
- UNC 926 hydrochloride
Catalog No.:BCC2445
CAS No.:1184136-10-4
- UNC1215
Catalog No.:BCC2023
CAS No.:1415800-43-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1314241-44-5 | SDF | Download SDF |
| PubChem ID | 46931242 | Appearance | Powder |
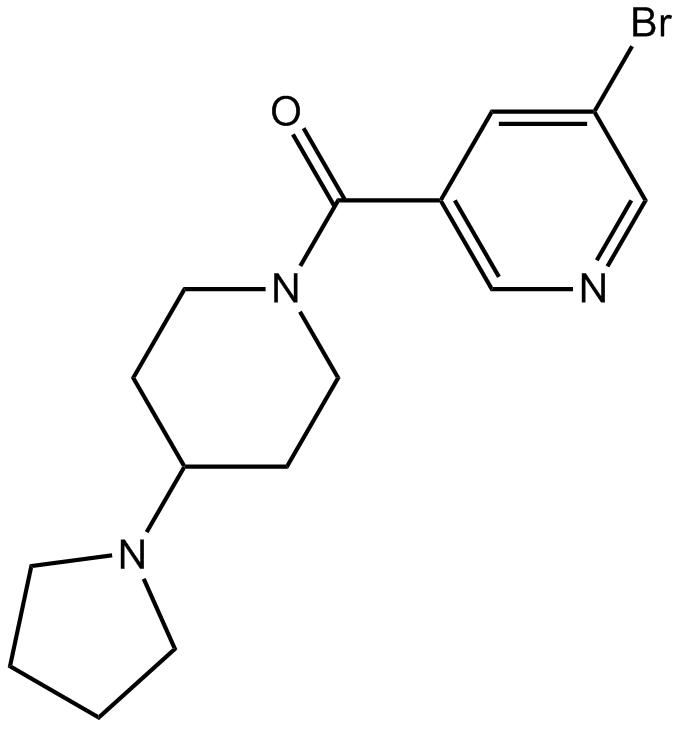
| Formula | C15H20BrN3O | M.Wt | 338.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (147.82 mM; Need ultrasonic) | ||
| Chemical Name | (5-bromopyridin-3-yl)-(4-pyrrolidin-1-ylpiperidin-1-yl)methanone | ||
| SMILES | C1CCN(C1)C2CCN(CC2)C(=O)C3=CC(=CN=C3)Br | ||
| Standard InChIKey | CQERVFFAOOUFEQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H20BrN3O/c16-13-9-12(10-17-11-13)15(20)19-7-3-14(4-8-19)18-5-1-2-6-18/h9-11,14H,1-8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | UNC669 is a potent antagonist of L3MBTL with IC50 values of 4.2 µM and 3.1 µM for L3MBTL1 and L3MBTL3, respectively. | |||||
| Targets | L3MBTL1 | L3MBTL3 | ||||
| IC50 | 4.2 µM | 3.1 µM | ||||

UNC669 Dilution Calculator

UNC669 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9565 mL | 14.7824 mL | 29.5648 mL | 59.1296 mL | 73.912 mL |
| 5 mM | 0.5913 mL | 2.9565 mL | 5.913 mL | 11.8259 mL | 14.7824 mL |
| 10 mM | 0.2956 mL | 1.4782 mL | 2.9565 mL | 5.913 mL | 7.3912 mL |
| 50 mM | 0.0591 mL | 0.2956 mL | 0.5913 mL | 1.1826 mL | 1.4782 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2956 mL | 0.5913 mL | 0.7391 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
UNC 669 is a potent antagonist of L3MBTL1 (IC50=4.2 μM) and L3MBTL3 (IC50=3.1 μM).
There is less bio-data supported for UNC669. UNC1679 is an analog of UNC669. UNC1215 is the first potent and selective antagonism of a methyl-lysine reader protein, L3MBTL3, which antagonizes the mono- and dimethyl-lysine reading function of L3MBTL3. [1]
Lysine methylation is a key epigenetic landmark, the dysregulation of which is related to many diseases. UNC1679 maintains in vitro and cellular potency with improved selectivity against other MBT-containing proteins. The antagonists described were also found to effectively interact with unlabeled endogenous L3MBTL3 in cells. [1]
Reference:
1. James LI, Korboukh VK, Krichevsky L et al. Small-molecule ligands of methyl-lysine binding proteins: optimization of selectivity for L3MBTL3. J Med Chem. 2013 Sep 26;56(18):7358-71. doi: 10.1021/jm400919p. Epub 2013 Sep 16.
- TC-N 22A
Catalog No.:BCC6150
CAS No.:1314140-00-5
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Boc-Ile-OH.1/2H2O
Catalog No.:BCC3406
CAS No.:13139-16-7
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- Boc-Trp-OH
Catalog No.:BCC3455
CAS No.:13139-14-5
- Teijin compound 1
Catalog No.:BCC6057
CAS No.:1313730-14-1
- Periplocin
Catalog No.:BCN2655
CAS No.:13137-64-9
- erythro-Guaiacylglycerol beta-threo-syringylglycerol ether
Catalog No.:BCN7333
CAS No.:1313434-74-0
- NVP-CGM097
Catalog No.:BCC5395
CAS No.:1313363-54-0
- Nystose
Catalog No.:BCN5397
CAS No.:13133-07-8
- threo Ifenprodil hemitartrate
Catalog No.:BCC7508
CAS No.:1312991-83-5
- Cercosporamide
Catalog No.:BCC2438
CAS No.:131436-22-1
- Amyloid Beta-Peptide (1-40) (human)
Catalog No.:BCC1045
CAS No.:131438-79-4
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- NG25
Catalog No.:BCC1799
CAS No.:1315355-93-1
- pep2-EVKI
Catalog No.:BCC5786
CAS No.:1315378-67-6
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
Design, synthesis, and protein methyltransferase activity of a unique set of constrained amine containing compounds.[Pubmed:27528434]
Bioorg Med Chem Lett. 2016 Sep 15;26(18):4436-4440.
Epigenetic alterations relate to various human diseases, and developing inhibitors of Kme regulatory proteins is considered to be a new frontier for drug discovery. We were inspired by the known multicyclic ligands, UNC669 and UNC926, which are the first reported small molecule ligands for a methyl-lysine binding domain. We hypothesized that reducing the conformational flexibility of the key amine moiety of UNC669 would result in a unique set of ligands. Twenty-five novel compounds containing a fused bi- or tricyclic amine or a spirocyclic amine were designed and synthesized. To gauge the potential of these amine-containing compounds to interact with Kme regulatory proteins, the compounds were screened against a panel of 24 protein methyltransferases. Compound 13 was discovered as a novel scaffold that interacts with SETD8 and could serve as a starting point for the future development of PKMT inhibitors.


