Bax inhibitor peptide, negative controlPeptide inhibit Bax translocation to mitochondria CAS# 1315378-74-5 |
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1315378-74-5 | SDF | Download SDF |
| PubChem ID | 24985487 | Appearance | Powder |
| Formula | C28H52N6O6S | M.Wt | 600.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
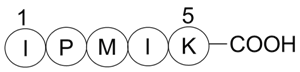
| Sequence | IPMIK | ||
| Chemical Name | (2S)-6-amino-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-1-[(2S,3S)-2-amino-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-4-methylsulfanylbutanoyl]amino]-3-methylpentanoyl]amino]hexanoic acid | ||
| SMILES | CCC(C)C(C(=O)N1CCCC1C(=O)NC(CCSC)C(=O)NC(C(C)CC)C(=O)NC(CCCCN)C(=O)O)N | ||
| Standard InChIKey | VRWAAYKVABJBAQ-FQJIPJFPSA-N | ||
| Standard InChI | InChI=1S/C28H52N6O6S/c1-6-17(3)22(30)27(38)34-15-10-12-21(34)25(36)31-19(13-16-41-5)24(35)33-23(18(4)7-2)26(37)32-20(28(39)40)11-8-9-14-29/h17-23H,6-16,29-30H2,1-5H3,(H,31,36)(H,32,37)(H,33,35)(H,39,40)/t17-,18-,19-,20-,21-,22-,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Negative control peptide for the Bax inhibitor peptide V5 P5, which inhibit Bax translocation to mitochondria and Bax-mediated apoptosis in vitro. |

Bax inhibitor peptide, negative control Dilution Calculator

Bax inhibitor peptide, negative control Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Negative control peptide for the Bax inhibitor peptides V5 and P5 , which inhibit Bax translocation to mitochondria and Bax-mediated apoptosis in vitro.
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- pep2-EVKI
Catalog No.:BCC5786
CAS No.:1315378-67-6
- NG25
Catalog No.:BCC1799
CAS No.:1315355-93-1
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- Amyloid Beta-Peptide (1-40) (human)
Catalog No.:BCC1045
CAS No.:131438-79-4
- Cercosporamide
Catalog No.:BCC2438
CAS No.:131436-22-1
- UNC669
Catalog No.:BCC3997
CAS No.:1314241-44-5
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- Triumbelletin
Catalog No.:BCN6779
CAS No.:131559-54-1
- Camelliaside B
Catalog No.:BCN3872
CAS No.:131573-90-5
- Calystegine A3
Catalog No.:BCN1884
CAS No.:131580-36-4
- Betulinaldehyde
Catalog No.:BCN1249
CAS No.:13159-28-9
- Amyloid Beta-peptide (25-35) (human)
Catalog No.:BCC1027
CAS No.:131602-53-4
- (+)-Pteryxin
Catalog No.:BCN3470
CAS No.:13161-75-6
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
Long non-coding RNA CCAT1 promotes human retinoblastoma SO-RB50 and Y79 cells through negative regulation of miR-218-5p.[Pubmed:28088735]
Biomed Pharmacother. 2017 Mar;87:683-691.
OBJECTIVE: To investigate the regulatory role and potential mechanism of long non-coding RNAs (lncRNA) in human retinoblastoma (RB). METHODS: The lncRNA profile in RB tissues were analyzed by microarray and quantitative reverse transcription PCR (qRT-PCR). One of the identified lncRNAs (LncRNA CCAT1) was selected for further experiments. SO-RB50 and Y79 cells were transfected with negative control, siRNA targeting lncRNA CCAT1 (si-CCAT1) and si-CCAT1+miR218-5p inhibitor, respectively. lncRNA CCAT1 expression was measured by qRT-PCR. Cell proliferation, migration and invasion were detected by CCK8, wound scratching, and transwell assay, respectively. Apoptosis and cell cycle distribution were assessed by flow cytometry. Apoptosis- (cle-caspase-3, cle-caspase-9, Bax and Bcl-2) and cell cycle-related protein expression (cyclin B1, CDC2 and p-CDC2 (Thr161)) were analyzed by Western blot. RESULTS: lncRNA CCAT1 expression in SO-RB50 and Y79 cells was significantly inhibited after si-CCAT1 transfection (P<0.01). Both RB cells exhibited significantly reduced proliferation, migration and invasion abilities, but markedly increased apoptosis at 48h after si-CCAT1 transfection (P<0.05 or 0.01). RB cells in si-CCAT1+miR218-5p inhibitor group had significantly higher proliferation, migration and invasion, but notably lower apoptosis compared with si-CCAT1 group at 24 and 48h after transfection (all P<0.05 or 0.01). si-CCAT1 significantly increased the expression of cle-caspase-3, cle-caspase-9, Bax, but decreased Bcl-2 expression (P<0.01). The proportion of G2/M SO-RB50 and Y79 cells in siCCAT1 group was significantly increased compared with negative control group (P<0.01). LncRNA CCAT1 interference significantly reduced the expression of cyclin B1, CDC2 and p-CDC2 (Thr161) (P<0.01). CONCLUSION: LncRNA CCAT1 promotes the proliferation migration and invasion, and reduces cell apoptosis of SO-RB50 and Y79 cells, probably through negative modulation of miR-218-5p. Our study suggested lncRNA CCAT1 as a potential biomarker and therapeutic target for RB.
Effect of curcumin and paclitaxel on breast carcinogenesis.[Pubmed:27779649]
Int J Oncol. 2016 Dec;49(6):2569-2577.
Global cancer burden increased to 14.1 million new cases in 2012; and breast cancer is the most common cancer in women worldwide, with nearly 1.7 million new cases diagnosed in 2012. Curcumin is the major bioactive ingredient extracted from the rhizome of the plant Curcuma longa (turmeric). Paclitaxel is a microtubule-stabilizing agent originally isolated from the bark of Taxus brevifolia. Curcumin and paclitaxel were evaluated with two human breast cancer cell lines as the luminal MCF-7 and the basal-like MDA-MB-231 that are either positive or negative for hormonal receptors estrogen receptor, progesterone receptor and HER2, respectively. Results indicated that curcumin combined with paclitaxel decreased c-Ha-Ras, Rho-A, p53 and Bcl-xL gene expression in comparison to control and substances alone in MCF-7 cell line. These two substances alone and combined decreased gene expression of Bcl-2 and NF-kappaB. However, CCND1 increased when both substances were combined in MCF-7 cells. Such substances decreased Bcl-2 and increased Bax protein expression. However, curcumin alone decreased IkappaBalpha and Stat-3 gene expression. Paclitaxel alone and combined increased IkappaBalpha and Stat-3. Curcumin alone and combined with paclitaxel increased p53, Bid, caspase-3, caspase-8 and Bax gene expression in MDA-MB-231, whereas Bcl-xL decreased such expression in MDA-MB-231 cells. When paclitaxel and curcumin were combined the expression of Bcl-2 protein was decreased. However, either substance alone and combined increased Bax protein expression corroborating the apoptotic effect of these substances. It can be concluded that curcumin may be of considerable value in synergistic therapy of breast cancer reducing the associated toxicity with use of drugs.
MicroRNA-34a regulates liver regeneration and the development of liver cancer in rats by targeting Notch signaling pathway.[Pubmed:28129650]
Oncotarget. 2017 Feb 21;8(8):13264-13276.
OBJECTIVE: This study aimed to investigate the role of microRNA-34a (miR-34a) in regulating liver regeneration (LR) and the development of liver cancer in rats by targeting Notch signaling pathway. METHODS: Thirty male Sprague-Dawley (SD) rats were randomly assigned into partial hepatectomy (PH) group and sham hepatectomy (SH) group. Hematoxylin and eosin (HE) staining was used to observe the histological change in liver tissues. Enzyme-linked immunosorbent assay (ELISA) was used to measure the serum tumor necrosis factor alpha (TNF-alpha) and interleukin-6 (IL-6) levels. Dual-luciferase reporter gene assay was performed to examine whether miR-34a targeted Notch1 gene. Human liver cancer Huh7 cells were transfected and divided into blank, negative control (NC), miR-34a mimics and miR-34a inhibitors groups. MTT and flow cytometry were used to detect cell growth, and cell cycle and apoptosis, respectively. Quantitative real-time polymerase chain reaction (qRT-PCR) was applied detect to the expressions of miR-34a and Notch receptor mRNA. Western blotting was performed to detect the protein expressions of Notch receptors, P21, Bax, Bcl-2 and Bcl-xL. Tumor xenograft in nude mice was done to observe tumor formation in different groups. RESULTS: Compared to the SH group, miR-34a expression in liver tissues in the PH group decreased first and then increased to the normal level during LR. In early stage of LR, the expressions of Notch receptors and miR-34a were negatively correlated. Compared to the blank and NC groups, the cell growth was inhibited, cell cycle was mainly arrested in the G2/M phase and cell apoptosis rate increased in the miR-34a mimics group. Moreover, the expressions of miR-34a, P21 and Bax were up-regulated, while the expressions of Notch receptors, and Bcl-2 and Bcl-xL were down-regulated in this group. Additionally, the tumor growth in the miR-34a mimics group was reduced. The miR-34a inhibitors group showed contrary tendencies. CONCLUSION: Our study demonstrates that miR-34a regulated LR and the development of liver cancer by inhibiting Notch signaling pathway.
Circulating microRNA-194 regulates human melanoma cells via PI3K/AKT/FoxO3a and p53/p21 signaling pathway.[Pubmed:28358423]
Oncol Rep. 2017 May;37(5):2702-2710.
In the present study, we analyzed the role of microRNA-194 circulating regulated human melanoma cell growth. We found that microRNA-194 expression was markedly suppressed in human melanoma patients, compared with negative control group. Next, disease-free survival (DFS) and overall survival (OS) of high expression in human melanoma patients was higher than those of low expression in human melanoma patients. MicroRNA-194 overexpression inhibited cell proliferation, induced apoptosis, increased caspase-3/-9 activities and promoted Bax/Bcl-2 of human melanoma cells. Furthermore, microRNA-194 overexpression also suppressed PI3K/AKT/FoxO3a signaling pathway and induced p53/p21 signaling pathway. PI3K inhibitor, suppressed PI3K, phosphorylation-AKT, FoxO3a protein expression and increased the effects of microRNA-194 overexpression on cell growth, apoptosis, caspase-3/-9 activities and Bax/Bcl-2 protein expression of human melanoma cells through the induction of p53/p21 signaling pathway. Taken together, these data indicate that circulating microRNA-194 regulated human melanoma cells via PI3K/AKT/FoxO3a and p53/p21 signaling pathway.
[The siRNA-mediated silencing of Bmi-1 promotes apoptosis and inhibits invasion of MCF-7 breast cancer cells].[Pubmed:27412932]
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016 Aug;32(8):1036-40.
Objective To investigate the effect of small interfering RNA (siRNA)-mediated silencing of the Bmi-1 gene on cell proliferation and invasion of MCF-7 human mammary carcinoma cell line and the potential molecular mechanisms. Methods Real-time quantitative PCR was used to detect the levels of Bmi-1 mRNA in the paired breast cancer and adjacent noncancerous breast tissues which were confirmed by pathological diagnosis. Bmi-1-siRNA was transfected into MCF-7 cells by a Lipofectamine(R) RNAiMAX transfection reagent. Flow cytometry was used to detect cell cycle and apoptosis of MCF-7 cells transfected by Bmi-1-siRNA. Western blotting was performed to detect the protein levels of P21, Bax and Bcl-2. Matrigel Transwell(TM) invasion assay was used to determine the cell invasion of MCF-7 cells with Bmi-1 silencing. The protein levels of E-cadherin, N-cadherin, vimentin were tested by Western blotting. Results The expression of Bmi-1 mRNA in the breast cancer tissues was higher than that in the adjacent noncancerous breast tissues. Bmi-1 silencing significantly suppressed the cell growth, arrested the cells in the G1/S phase and promoted the apoptosis of MCF-7 cells. Compared with blank control group or negative control group, the Bmi-1-silenced group showed the increased expressions of P21 and Bax and the decreased expression of Bcl-2. In addition, Bmi-1 silencing significantly suppressed the cell invasion and promoted the expression of E-cadherin as well as downregulated the expressions of N-cadherin and vimentin in MCF-7 cells. Conclusion The invasion of MCF-7 cells can be inhibited by Bmi-1 silencing, of which the molecular regulation mechanism might be associated with the inhibition of tumor cell epithelial-mesenchymal transition.
The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo.[Pubmed:16400007]
Am J Pathol. 2006 Jan;168(1):33-41.
Inflammatory cell recruitment, activation, and apoptosis are highly regulated processes involving several checkpoints controlling the resolution of inflammation. We investigated the role of the mitogen-activated protein kinase (MAPK) signaling pathway (ie, ERK1/2) and apoptosis-regulating Bcl-2 family members (ie, Bcl-x(L) and Bax) in the resolution of a rat carrageenan-induced pleurisy model. The specific ERK1/2 inhibitor PD98059 enhanced the resolution of inflammation, whereas the MEK1/2 inhibitor U0126 had no effect and the flavonoid apigenin, a nonspecific inhibitor of ERK1/2 and COX-2, augmented inflammation. Specifically, PD98059 significantly decreased the total number of macrophages and neutrophils in the pleural cavity, mainly by increasing the rate of neutrophil apoptosis, as measured by Annexin V labeling and morphological analysis. Conversely, a specific inhibitor of proapoptotic Bax (V5) increased inflammation, indicating that by preventing apoptosis in vivo, resolution of inflammation is delayed. This was associated with a decrease in neutrophil apoptosis and an increase in macrophage and neutrophil numbers perpetuating the inflammatory response. In conclusion, this study shows that ERK1/2, Bax, and Bcl-x(L) play important functional roles in the resolution phase of the acute inflammatory response in vivo by influencing apoptosis. Importantly, these data may provide novel therapeutic targets for the treatment of inflammatory diseases.
Bax-inhibiting peptide derived from mouse and rat Ku70.[Pubmed:15358121]
Biochem Biophys Res Commun. 2004 Sep 3;321(4):961-6.
Bax is a proapoptotic protein that plays a key role in the induction of apoptosis. Ku70 has activities to repair DNA damage in the nucleus and to suppress apoptosis by inhibiting Bax in the cytosol. We previously designed peptides based on the amino acid sequence of Bax-binding domain of human Ku70, and showed that these peptides bind Bax and inhibit cell death in human cell lines. In the present report, we examined the biological activities of other pentapeptides, VPTLK and VPALR, derived from mouse and rat Ku70. Cells in culture accumulated FITC-labeled VPTLK and VPALR, indicating that these peptides are cell permeable (human, mouse, rat, and porcine cells were examined). These peptides bound to Bax and suppressed cell death in various cell types including primary cultured cells. These data suggest that such Bax inhibiting peptides from three mammalian species may be used to protect healthy cells from apoptotic injury under pathological conditions.


