Bavisant dihydrochloride hydrateHuman H3 receptor antagonist CAS# 1103522-80-0 |
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1103522-80-0 | SDF | Download SDF |
| PubChem ID | 56843503 | Appearance | Powder |
| Formula | C19H31Cl2N3O3 | M.Wt | 420.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (118.94 mM) DMSO : ≥ 1 mg/mL (2.38 mM) *"≥" means soluble, but saturation unknown. | ||
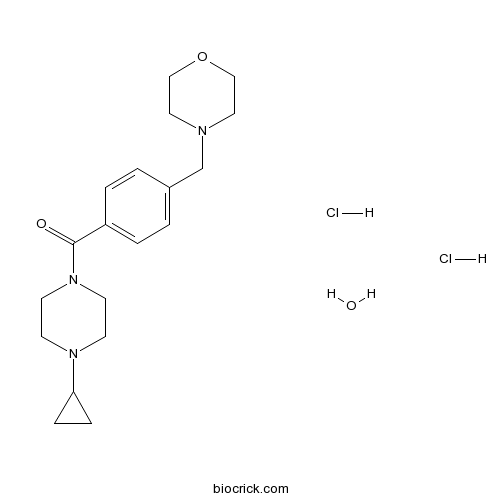
| Chemical Name | (4-cyclopropylpiperazin-1-yl)-[4-(morpholin-4-ylmethyl)phenyl]methanone;hydrate;dihydrochloride | ||
| SMILES | C1CC1N2CCN(CC2)C(=O)C3=CC=C(C=C3)CN4CCOCC4.O.Cl.Cl | ||
| Standard InChIKey | BLFBQJUVAGIUBL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H27N3O2.2ClH.H2O/c23-19(22-9-7-21(8-10-22)18-5-6-18)17-3-1-16(2-4-17)15-20-11-13-24-14-12-20;;;/h1-4,18H,5-15H2;2*1H;1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bavisant dihydrochloride hydrate Dilution Calculator

Bavisant dihydrochloride hydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3789 mL | 11.8943 mL | 23.7886 mL | 47.5771 mL | 59.4714 mL |
| 5 mM | 0.4758 mL | 2.3789 mL | 4.7577 mL | 9.5154 mL | 11.8943 mL |
| 10 mM | 0.2379 mL | 1.1894 mL | 2.3789 mL | 4.7577 mL | 5.9471 mL |
| 50 mM | 0.0476 mL | 0.2379 mL | 0.4758 mL | 0.9515 mL | 1.1894 mL |
| 100 mM | 0.0238 mL | 0.1189 mL | 0.2379 mL | 0.4758 mL | 0.5947 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bavisant dihydrochloride hydrate is a highly selective, orally active antagonist of the human H3 receptor [1].
The human H3 receptor is a G-protein coupled receptor, which is expressed in the central nervous system and to a lesser extent the peripheral nervous system. Histamine H3 receptors regulate the release of other neurotransmitters [1].
Bavisant dihydrochloride hydrate is an active antagonist of the human H3 receptor with a novel mechanism of action, involving wakefulness and cognition, with potential as a treatment for ADHD. In adults with ADHD, three dosages of bavisant (1 mg/day, 3 mg/day or 10mg/day) were evaluated. Compared with the placebo group (-8.8) in the total ADHD-RS-IV score at day 42 (primary efficacy endpoint), the scores were -9.3, -11.2 and -12.2 in the 1mg/day, 3mg/day and 10mg/day groups, respectively. The changes were not statistically superior to placebo. However, mean change from baseline was superior to placebo in the atomoxetine (-15.3) and OROS methylphenidate (-15.7) groups (p < 0.005). The two lower dosages showed a good tolerability profile [1].
References:
[1]. Weisler RH, Pandina GJ, Daly EJ, et al. Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs, 2012, 26(5): 421-434.
- CGS 19755
Catalog No.:BCC6986
CAS No.:110347-85-8
- FD-838
Catalog No.:BCN6396
CAS No.:110341-78-1
- α-Bungarotoxin
Catalog No.:BCC7264
CAS No.:11032-79-4
- Santalol
Catalog No.:BCN8352
CAS No.:11031-45-1
- CI 966 hydrochloride
Catalog No.:BCC7010
CAS No.:110283-66-4
- Bacoside A
Catalog No.:BCC8127
CAS No.:11028-00-5
- Agnuside
Catalog No.:BCN5990
CAS No.:11027-63-7
- Amrubicin
Catalog No.:BCC3640
CAS No.:110267-81-7
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Digitonin
Catalog No.:BCN3734
CAS No.:11024-24-1
- S 32826
Catalog No.:BCC7678
CAS No.:1103672-43-0
- Ch 55
Catalog No.:BCC7241
CAS No.:110368-33-7
- 3-O-Methyltagitinin F
Catalog No.:BCN5991
CAS No.:110382-37-1
- 2,3-Dihydroheveaflavone
Catalog No.:BCN4019
CAS No.:110382-42-8
- Meclizine hydrochloride
Catalog No.:BCC9017
CAS No.:1104-22-9
- Higenamine HCl
Catalog No.:BCN2831
CAS No.:11041-94-4
- Gelsemiol
Catalog No.:BCN5992
CAS No.:110414-77-2
- Dolastatin 10
Catalog No.:BCC4056
CAS No.:110417-88-4
- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- Crotastriatine
Catalog No.:BCN2101
CAS No.:11051-94-8
- 6,11-Di-O-acetylalbrassitriol
Catalog No.:BCN7273
CAS No.:110538-20-0
Drug repositioning as a route to anti-malarial drug discovery: preliminary investigation of the in vitro anti-malarial efficacy of emetine dihydrochloride hydrate.[Pubmed:24107123]
Malar J. 2013 Oct 9;12:359.
BACKGROUND: Drug repurposing or repositioning refers to the usage of existing drugs in diseases other than those it was originally used for. For diseases like malaria, where there is an urgent need for active drug candidates, the strategy offers a route to significantly shorten the traditional drug development pipelines. Preliminary high-throughput screens on patent expired drug libraries have recently been carried out for Plasmodium falciparum. This study reports the systematic and objective further interrogation of selected compounds reported in these studies, to enable their repositioning as novel stand-alone anti-malarials or as combinatorial partners. METHODS: SYBR Green flow cytometry and micro-titre plate assays optimized in the laboratory were used to monitor drug susceptibility of in vitro cultures of P. falciparum K1 parasite strains. Previously described fixed-ratio methods were adopted to investigate drug interactions. RESULTS: Emetine dihydrochloride hydrate, an anti-protozoal drug previously used for intestinal and tissue amoebiasis was shown to have potent inhibitory properties (IC(5)(0) doses of ~ 47 nM) in the multidrug resistant K1 strain of P. falciparum. The sum 50% fractional inhibitory concentration ( summation operatorFIC(5)(0), (9)(0)) of the interaction of emetine dihydrochloride hydrate and dihydroartemisinin against the K1 strains of P. falciparum ranged from 0.88-1.48. CONCLUSION: The results warrant further investigation of emetine dihydrochloride hydrate as a potential stand-alone anti-malarial option. The interaction between the drug and the current front line dihydroartemisinin ranged from additive to mildly antagonistic in the fixed drug ratios tested.
Inhibition of dystrophin breakdown and endothelial nitric-oxide synthase uncoupling accounts for cytoprotection by 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazo lylmethyl)-1H-indazole dihydrochloride 3.5 hydrate (DY-9760e) in left ventricular hypertrophied Mice.[Pubmed:19889795]
J Pharmacol Exp Ther. 2010 Feb;332(2):421-8.
Using a heart ischemia/reperfusion model in rats, we recently demonstrated that 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazo lylmethyl)-1H-indazole dihydrochloride 3.5 hydrate (DY-9760e), a calmodulin inhibitor, is a cardioprotective drug. Here, we examined cardioprotective mechanisms of DY-9760e in hypertrophy and heart failure using a mouse transverse aortic constriction (TAC) model. Mice were subjected to TAC and 2 weeks later they were administered DY-9760e for another 6 weeks (at 10 or 20 mg/kg/day p.o.). Chronic administration inhibited TAC-induced increased heart-to-body weight ratio dose-dependently. Consistent with inhibition of hypertrophy, fraction shortening, an indicator of heart contractile function, assessed by echocardiography was completely restored by DY-9760e (20 mg/kg/day) administration. Inhibition of TAC-induced atrial natriuretic peptide (ANP) up-regulation further confirmed an antihypertrophic effect of DY-9760e. It is noteworthy that we found that breakdown of dystrophin and spectrin by calpain was associated with heart failure in TAC mice. Caveolin-3 breakdown was closely associated with endothelial nitric-oxide synthase (eNOS) dissociation from the plasma membrane and its subsequent uncoupling. Uncoupled monomeric eNOS formation was associated with increased protein tyrosine nitration, suggesting peroxynitrite production and NO and superoxide formation. It is important to note that 6 weeks of DY-9760e treatment significantly blocked hypertrophic responses, such as increased heart weight and ANP induction. Overall, we show that inhibition of both dystrophin/spectrin breakdown and uncoupling of eNOS probably underlies the cardioprotective mechanisms of DY-9760e. The observed protection of sarcolemmal proteins and eNOS by DY-9760e during pressure overload suggests a novel therapeutic strategy to rescue the heart from hypertrophy-induced failure.
In vitro metabolism of the calmodulin antagonist DY-9760e (3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidaz olylmethyl)-1H-indazole dihydrochloride 3.5 hydrate) by human liver microsomes: involvement of cytochromes p450 in atypical kinetics and potential drug interactions.[Pubmed:16049129]
Drug Metab Dispos. 2005 Nov;33(11):1628-36.
Human cytochrome P450 (P450) isozyme(s) responsible for metabolism of the calmodulin antagonist 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazo lylmethyl)-1H-indazole dihydrochloride 3.5 hydrate (DY-9760e) and kinetic profiles for formation of its six primary metabolites [M3, M5, M6, M7, M8, and DY-9836 (3-[2-[4-(3-chloro-2-methylphenyl)piperazinyl]ethyl]-5,6-dimethoxyindazole)] were identified using human liver microsomes and recombinant P450 enzymes. In vitro experiments, including an immunoinhibition study, correlation analysis, and reactions with recombinant P450 enzymes, revealed that CYP3A4 is the primary P450 isozyme responsible for the formation of the DY-9760e metabolites, except for M5, which is metabolized by CYP2C9. Additionally, at clinically relevant concentrations, CYP2C8 and 2C19 make some contribution to the formation of M3 and M5, respectively. The formation rates of DY-9760e metabolites except for M8 by human liver microsomes are not consistent with a Michaelis-Menten kinetics model, but are better described by a substrate inhibition model. In contrast, the enzyme kinetics for all metabolites formed by recombinant CYP3A4 can be described by an autoactivation model or a mixed model of autoactivation and biphasic kinetics. Inhibition of human P450 enzymes by DY-9760e in human liver microsomes was also investigated. DY-9760e is a very potent competitive inhibitor of CYP2C8, 2C9 and 2D6 (Ki 0.25-1.7 microM), a mixed competitive and noncompetitive inhibitor of CYP2C19 (Ki 2.4 microM) and a moderate inhibitor of CYP1A2 and 3A4 (Ki 11.4-20.1 microM), suggesting a high possibility for human drug-drug interaction.
Investigating antimalarial drug interactions of emetine dihydrochloride hydrate using CalcuSyn-based interactivity calculations.[Pubmed:28257497]
PLoS One. 2017 Mar 3;12(3):e0173303.
The widespread introduction of artemisinin-based combination therapy has contributed to recent reductions in malaria mortality. Combination therapies have a range of advantages, including synergism, toxicity reduction, and delaying the onset of resistance acquisition. Unfortunately, antimalarial combination therapy is limited by the depleting repertoire of effective drugs with distinct target pathways. To fast-track antimalarial drug discovery, we have previously employed drug-repositioning to identify the anti-amoebic drug, emetine dihydrochloride hydrate, as a potential candidate for repositioned use against malaria. Despite its 1000-fold increase in in vitro antimalarial potency (ED50 47 nM) compared with its anti-amoebic potency (ED50 26-32 uM), practical use of the compound has been limited by dose-dependent toxicity (emesis and cardiotoxicity). Identification of a synergistic partner drug would present an opportunity for dose-reduction, thus increasing the therapeutic window. The lack of reliable and standardised methodology to enable the in vitro definition of synergistic potential for antimalarials is a major drawback. Here we use isobologram and combination-index data generated by CalcuSyn software analyses (Biosoft v2.1) to define drug interactivity in an objective, automated manner. The method, based on the median effect principle proposed by Chou and Talalay, was initially validated for antimalarial application using the known synergistic combination (atovaquone-proguanil). The combination was used to further understand the relationship between SYBR Green viability and cytocidal versus cytostatic effects of drugs at higher levels of inhibition. We report here the use of the optimised Chou Talalay method to define synergistic antimalarial drug interactivity between emetine dihydrochloride hydrate and atovaquone. The novel findings present a potential route to harness the nanomolar antimalarial efficacy of this affordable natural product.


