S 32826Potent autotaxin inhibitor CAS# 1103672-43-0 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1103672-43-0 | SDF | Download SDF |
| PubChem ID | 78243746 | Appearance | Powder |
| Formula | C21H34NO4PNa2 | M.Wt | 441.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
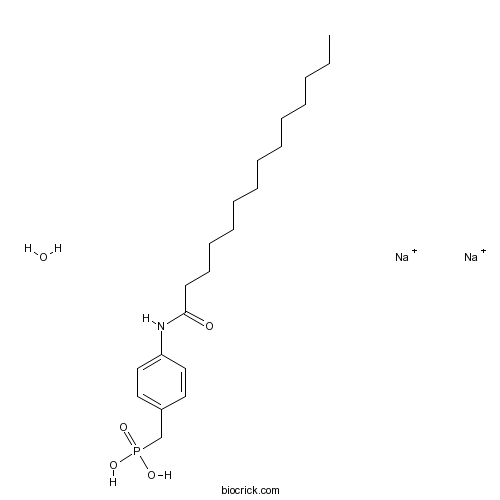
| Chemical Name | disodium;[4-(tetradecanoylamino)phenyl]methylphosphonic acid;hydrate | ||
| SMILES | CCCCCCCCCCCCCC(=O)NC1=CC=C(C=C1)CP(=O)(O)O.O.[Na+].[Na+] | ||
| Standard InChIKey | KUYQLJWFYWGYNJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H36NO4P.2Na.H2O/c1-2-3-4-5-6-7-8-9-10-11-12-13-21(23)22-20-16-14-19(15-17-20)18-27(24,25)26;;;/h14-17H,2-13,18H2,1H3,(H,22,23)(H2,24,25,26);;;1H2/q;2*+1; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of autotaxin (IC50 = 9 nM). Displays similar inhibitory effects at all three autotaxin isoforms (α, β and γ). Exhibits no affinity for lysophosphatidic acid receptor 1 (LPA1) at concentrations up to 10 μM. Inhibits LPA release from adipocytes (IC50 = 90 nM). |

S 32826 Dilution Calculator

S 32826 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2653 mL | 11.3263 mL | 22.6526 mL | 45.3052 mL | 56.6316 mL |
| 5 mM | 0.4531 mL | 2.2653 mL | 4.5305 mL | 9.061 mL | 11.3263 mL |
| 10 mM | 0.2265 mL | 1.1326 mL | 2.2653 mL | 4.5305 mL | 5.6632 mL |
| 50 mM | 0.0453 mL | 0.2265 mL | 0.4531 mL | 0.9061 mL | 1.1326 mL |
| 100 mM | 0.0227 mL | 0.1133 mL | 0.2265 mL | 0.4531 mL | 0.5663 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- CGS 19755
Catalog No.:BCC6986
CAS No.:110347-85-8
- FD-838
Catalog No.:BCN6396
CAS No.:110341-78-1
- α-Bungarotoxin
Catalog No.:BCC7264
CAS No.:11032-79-4
- Santalol
Catalog No.:BCN8352
CAS No.:11031-45-1
- CI 966 hydrochloride
Catalog No.:BCC7010
CAS No.:110283-66-4
- Bacoside A
Catalog No.:BCC8127
CAS No.:11028-00-5
- Agnuside
Catalog No.:BCN5990
CAS No.:11027-63-7
- Amrubicin
Catalog No.:BCC3640
CAS No.:110267-81-7
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Ch 55
Catalog No.:BCC7241
CAS No.:110368-33-7
- 3-O-Methyltagitinin F
Catalog No.:BCN5991
CAS No.:110382-37-1
- 2,3-Dihydroheveaflavone
Catalog No.:BCN4019
CAS No.:110382-42-8
- Meclizine hydrochloride
Catalog No.:BCC9017
CAS No.:1104-22-9
- Higenamine HCl
Catalog No.:BCN2831
CAS No.:11041-94-4
- Gelsemiol
Catalog No.:BCN5992
CAS No.:110414-77-2
- Dolastatin 10
Catalog No.:BCC4056
CAS No.:110417-88-4
- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- Crotastriatine
Catalog No.:BCN2101
CAS No.:11051-94-8
- 6,11-Di-O-acetylalbrassitriol
Catalog No.:BCN7273
CAS No.:110538-20-0
- Scutebarbatine F
Catalog No.:BCN5377
CAS No.:910099-78-4
Airborne measurements of isoprene and monoterpene emissions from southeastern U.S. forests.[Pubmed:28384571]
Sci Total Environ. 2017 Oct 1;595:149-158.
Isoprene and monoterpene emission rates are essential inputs for atmospheric chemistry models that simulate atmospheric oxidant and particle distributions. Process studies of the biochemical and physiological mechanisms controlling these emissions are advancing our understanding and the accuracy of model predictions but efforts to quantify regional emissions have been limited by a lack of constraints on regional distributions of ecosystem emission capacities. We used an airborne wavelet-based eddy covariance measurement technique to characterize isoprene and monoterpene fluxes with high spatial resolution during the 2013 SAS (Southeast Atmosphere Study) in the southeastern United States. The fluxes measured by direct eddy covariance were comparable to emissions independently estimated using an indirect inverse modeling approach. Isoprene emission factors based on the aircraft wavelet flux estimates for high isoprene chemotypes (e.g., oaks) were similar to the MEGAN2.1 biogenic emission model estimates for landscapes dominated by oaks. Aircraft flux measurement estimates for landscapes with fewer isoprene emitting trees (e.g., pine plantations), were about a factor of two lower than MEGAN2.1 model estimates. The tendency for high isoprene emitters in these landscapes to occur in the shaded understory, where light dependent isoprene emissions are diminished, may explain the lower than expected emissions. This result demonstrates the importance of accurately representing the vertical profile of isoprene emitting biomass in biogenic emission models. Airborne measurement-based emission factors for high monoterpene chemotypes agreed with MEGAN2.1 in landscapes dominated by pine (high monoterpene chemotype) trees but were more than a factor of three higher than model estimates for landscapes dominated by oak (relatively low monoterpene emitting) trees. This results suggests that unaccounted processes, such as floral emissions or light dependent monoterpene emissions, or vegetation other than high monoterpene emitting trees may be an important source of monoterpene emissions in those landscapes and should be identified and included in biogenic emission models.
Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016.[Pubmed:28384133]
MMWR Morb Mortal Wkly Rep. 2017 Apr 7;66(13):366-373.
BACKGROUND: In collaboration with state, tribal, local, and territorial health departments, CDC established the U.S. Zika Pregnancy Registry (USZPR) in early 2016 to monitor pregnant women with laboratory evidence of possible recent Zika virus infection and their infants. METHODS: This report includes an analysis of completed pregnancies (which include live births and pregnancy losses, regardless of gestational age) in the 50 U.S. states and the District of Columbia (DC) with laboratory evidence of possible recent Zika virus infection reported to the USZPR from January 15 to December 27, 2016. Birth defects potentially associated with Zika virus infection during pregnancy include brain abnormalities and/or microcephaly, eye abnormalities, other consequences of central nervous system dysfunction, and neural tube defects and other early brain malformations. RESULTS: During the analysis period, 1,297 pregnant women in 44 states were reported to the USZPR. Zika virus-associated birth defects were reported for 51 (5%) of the 972 fetuses/infants from completed pregnancies with laboratory evidence of possible recent Zika virus infection (95% confidence interval [CI] = 4%-7%); the proportion was higher when restricted to pregnancies with laboratory-confirmed Zika virus infection (24/250 completed pregnancies [10%, 95% CI = 7%-14%]). Birth defects were reported in 15% (95% CI = 8%-26%) of fetuses/infants of completed pregnancies with confirmed Zika virus infection in the first trimester. Among 895 liveborn infants from pregnancies with possible recent Zika virus infection, postnatal neuroimaging was reported for 221 (25%), and Zika virus testing of at least one infant specimen was reported for 585 (65%). CONCLUSIONS AND IMPLICATIONS FOR PUBLIC HEALTH PRACTICE: These findings highlight why pregnant women should avoid Zika virus exposure. Because the full clinical spectrum of congenital Zika virus infection is not yet known, all infants born to women with laboratory evidence of possible recent Zika virus infection during pregnancy should receive postnatal neuroimaging and Zika virus testing in addition to a comprehensive newborn physical exam and hearing screen. Identification and follow-up care of infants born to women with laboratory evidence of possible recent Zika virus infection during pregnancy and infants with possible congenital Zika virus infection can ensure that appropriate clinical services are available.
Neuropeptide S reduces duodenal bicarbonate secretion and ethanol-induced increases in duodenal motility in rats.[Pubmed:28384243]
PLoS One. 2017 Apr 6;12(4):e0175312.
Alcohol disrupts the intestinal mucosal barrier by inducing metabolic and functional changes in epithelial cells. Recently, we showed that neuropeptide S (NPS) decreases duodenal motility and increases mucosal paracellular permeability, suggesting a role of NPS in the pathogenesis of disorders and dysfunctions in the small intestine. The aim of the present study was to investigate the effects of NPS on ethanol- and HCl-induced changes of duodenal mucosal barrier function and motility. Rats were anaesthetized with thiobarbiturate, and a 30-mm segment of the proximal duodenum with an intact blood supply was perfused in situ. The effects on duodenal bicarbonate secretion, the blood-to-lumen clearance of 51Cr-EDTA, motility and transepithelial net fluid flux were investigated. Intravenous (i.v.) administration of NPS significantly reduced duodenal mucosal bicarbonate secretion and stimulated mucosal transepithelial fluid absorption, mechanisms dependent on nitrergic signaling. NPS dose-dependently reduced ethanol-induced increases in duodenal motility. NPS (83 pmol.kg-1.min-1, i.v.) reduced the bicarbonate and fluid secretory response to luminal ethanol, whereas a 10-fold higher dose stimulated fluid secretion but did not influence bicarbonate secretion. In NPS-treated animals, duodenal perfusion of acid (pH 3) induced greater bicarbonate secretory rates than in controls. Pre-treating animals with Nomega-nitro-L-arginine methyl ester (L-NAME) inhibited the effect of NPS on bicarbonate secretion. In response to luminal acid, NPS-treated animals had significantly higher paracellular permeability compared to controls, an effect that was abolished by L-NAME. Our findings demonstrate that NPS reduces basal and ethanol-induced increases in duodenal motility. In addition, NPS increases luminal alkalinization and mucosal permeability in response to luminal acid via mechanisms that are dependent on nitric oxide signaling. The data support a role for NPS in neurohumoral regulation of duodenal mucosal barrier function and motility.
Autotaxin is induced by TSA through HDAC3 and HDAC7 inhibition and antagonizes the TSA-induced cell apoptosis.[Pubmed:21314984]
Mol Cancer. 2011 Feb 12;10:18.
BACKGROUND: Autotaxin (ATX) is a secreted glycoprotein with the lysophospholipase D (lysoPLD) activity to convert lysophosphatidylcholine (LPC) into lysophosphatidic acid (LPA), a bioactive lysophospholipid involved in diverse biological actions. ATX is highly expressed in some cancer cells and contributes to their tumorigenesis, invasion, and metastases, while in other cancer cells ATX is silenced or expressed at low level. The mechanism of ATX expression regulation in cancer cells remains largely unknown. RESULTS: In the present study, we demonstrated that trichostatin A (TSA), a well-known HDAC inhibitor (HDACi), significantly induced ATX expression in SW480 and several other cancer cells with low or undetectable endogenous ATX expression. ATX induction could be observed when HDAC3 and HDAC7 were down-regulated by their siRNAs. It was found that HDAC7 expression levels were low in the cancer cells with high endogenous ATX expression. Exogenous over-expression of HDAC7 inhibited ATX expression in these cells in a HDAC3-dependent manner. These data indicate that HDAC3 and HDAC7 collaboratively suppress ATX expression in cancer cells, and suggest that TSA induce ATX expression by inhibiting HDAC3 and HDAC7. The biological significance of this regulation mechanism was revealed by demonstrating that TSA-induced ATX protected cancer cells against TSA-induced apoptosis by producing LPA through its lysoPLD activity, which could be reversed by BrP-LPA and S32826, the inhibitors of the ATX-LPA axis. CONCLUSIONS: We have demonstrated that ATX expression is repressed by HDAC3 and HDAC7 in cancer cells. During TSA treatment, ATX is induced due to the HDAC3 and HDAC7 inhibition and functionally antagonizes the TSA-induced apoptosis. These results reveal an internal HDACi-resistant mechanism in cancer cells, and suggest that the inhibition of ATX-LPA axis would be helpful to improve the efficacy of HDACi-based therapeutics against cancer.
S32826, a nanomolar inhibitor of autotaxin: discovery, synthesis and applications as a pharmacological tool.[Pubmed:18755937]
J Pharmacol Exp Ther. 2008 Dec;327(3):809-19.
Autotaxin catalyzes the transformation of lyso-phosphatidylcholine in lyso-phosphatidic acid (LPA). LPA is a phospholipid possessing a large panel of activity, in particular as a motility factor or as a growth signal, through its G-protein coupled seven transmembrane receptors. Indirect evidence strongly suggests that autotaxin is the main, if not the only source of circulating LPA. Because of its central role in pathologic conditions, such as oncology and diabetes/obesity, the biochemical properties of autotaxin has attracted a lot of attention, but confirmation of its role in pathology remains elusive. One way to validate and/or confirm its central role, is to find potent and selective inhibitors. A systematic screening of several thousand compounds using a colorimetric assay and taking advantage of the phosphodiesterase activity of autotaxin that requires the enzymatic site than for LPA generation, led to the discovery of a potent nanomolar inhibitor, [4-(tetradecanoylamino)benzyl]phosphonic acid (S32826). This compound was inhibitory toward the various autotaxin isoforms, using an assay measuring the [(14)C]lyso-phosphatidylcholine conversion into [(14)C]LPA. We also evaluated the activity of S32826 in cellular models of diabesity and oncology. Nevertheless, the poor in vivo stability and/or bioavailability of the compound did not permit to use it in animals. S32826 is the first reported inhibitor of autotaxin with an IC(50) in the nanomolar range that can be used to validate the role of autotaxin in various pathologies in cellular models.


