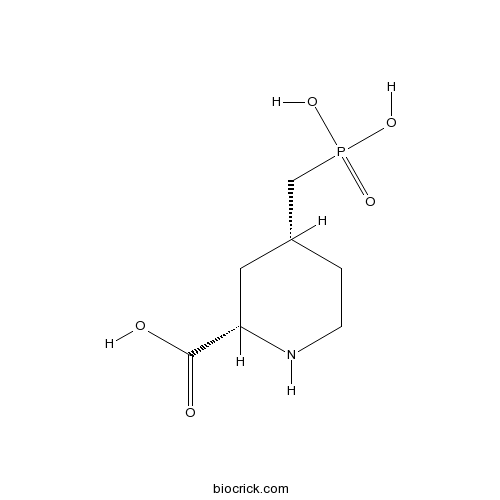
CGS 19755CAS# 110347-85-8 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110347-85-8 | SDF | Download SDF |
| PubChem ID | 68736 | Appearance | Powder |
| Formula | C7H14NO5P | M.Wt | 223.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | (2S,4R)-4-(phosphonomethyl)piperidine-2-carboxylic acid | ||
| SMILES | C1CNC(CC1CP(=O)(O)O)C(=O)O | ||
| Standard InChIKey | LPMRCCNDNGONCD-RITPCOANSA-N | ||
| Standard InChI | InChI=1S/C7H14NO5P/c9-7(10)6-3-5(1-2-8-6)4-14(11,12)13/h5-6,8H,1-4H2,(H,9,10)(H2,11,12,13)/t5-,6+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, competitive NMDA receptor antagonist. Anticonvulsant and neuroprotective. Also available as part of the Mixed NMDA Receptor. |

CGS 19755 Dilution Calculator

CGS 19755 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4809 mL | 22.4044 mL | 44.8089 mL | 89.6178 mL | 112.0222 mL |
| 5 mM | 0.8962 mL | 4.4809 mL | 8.9618 mL | 17.9236 mL | 22.4044 mL |
| 10 mM | 0.4481 mL | 2.2404 mL | 4.4809 mL | 8.9618 mL | 11.2022 mL |
| 50 mM | 0.0896 mL | 0.4481 mL | 0.8962 mL | 1.7924 mL | 2.2404 mL |
| 100 mM | 0.0448 mL | 0.224 mL | 0.4481 mL | 0.8962 mL | 1.1202 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- FD-838
Catalog No.:BCN6396
CAS No.:110341-78-1
- α-Bungarotoxin
Catalog No.:BCC7264
CAS No.:11032-79-4
- Santalol
Catalog No.:BCN8352
CAS No.:11031-45-1
- CI 966 hydrochloride
Catalog No.:BCC7010
CAS No.:110283-66-4
- Bacoside A
Catalog No.:BCC8127
CAS No.:11028-00-5
- Agnuside
Catalog No.:BCN5990
CAS No.:11027-63-7
- Amrubicin
Catalog No.:BCC3640
CAS No.:110267-81-7
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Digitonin
Catalog No.:BCN3734
CAS No.:11024-24-1
- Nothofagin
Catalog No.:BCN3787
CAS No.:11023-94-2
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- S 32826
Catalog No.:BCC7678
CAS No.:1103672-43-0
- Ch 55
Catalog No.:BCC7241
CAS No.:110368-33-7
- 3-O-Methyltagitinin F
Catalog No.:BCN5991
CAS No.:110382-37-1
- 2,3-Dihydroheveaflavone
Catalog No.:BCN4019
CAS No.:110382-42-8
- Meclizine hydrochloride
Catalog No.:BCC9017
CAS No.:1104-22-9
- Higenamine HCl
Catalog No.:BCN2831
CAS No.:11041-94-4
- Gelsemiol
Catalog No.:BCN5992
CAS No.:110414-77-2
- Dolastatin 10
Catalog No.:BCC4056
CAS No.:110417-88-4
- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- Crotastriatine
Catalog No.:BCN2101
CAS No.:11051-94-8
Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators.[Pubmed:10541229]
J Neurosurg. 1999 Nov;91(5):737-43.
OBJECT: Excessive activity of excitatory amino acids released after head trauma has been demonstrated to contribute to progressive injury in animal models and human studies. Several pharmacological agents that act as antagonists to the glutamate receptor have shown promise in limiting this progression. The efficacy of the N-methyl-D-aspartate receptor antagonist Selfotel (CGS 19755) was evaluated in two parallel studies of severely head injured patients, defined as patients with post resuscitation Glasgow Coma Scale scores of 4 to 8. METHODS: A total of 693 patients were prospectively enrolled in two multicenter double-blind studies. Comparison between the treatment groups showed no significant difference with regard to demographic data, previous incidence of hypotension, and severity of injury. As the study progressed, the Safety and Monitoring Committee became concerned about possible increased deaths and serious brain-related adverse events in the treatment arm of the two head injury trials, as well as deaths in the two stroke trials being monitored concurrently. The Selfotel trials were stopped prematurely because of this concern and because an interim efficacy analysis indicated that the likelihood of demonstrating success with the agent if the studies had been completed was almost nil. CONCLUSIONS: Subsequently, more complete data analysis revealed no statistically significant difference in mortality rates in all cases between the two treatment groups in the head injury trials. In this report the authors examine the studies in detail and discuss the potential application of the data to future trial designs.
First observations of the safety and tolerability of a competitive antagonist to the glutamate NMDA receptor (CGS 19755) in patients with severe head injury.[Pubmed:10521143]
J Neurotrauma. 1999 Sep;16(9):843-50.
A dose escalation, safety, and tolerability study of a competitive antagonist to the N-methyl-D-aspartate (NMDA) glutamate receptor (CGS 19755, Selfotel) in patients with severe head injury is reported. The drug was administered i.v. on two separate occasions, 24 h apart, to 31 patients. The dosage was escalated during the study from 1 mg/kg to 6 mg/kg. Continuous monitoring of mean arterial pressure (MABP), intracranial pressure (ICP), cerebral pressure (CPP), arterial oxygen saturation (SaO2), jugular bulb oxygen saturation (SjO2), and temperature was performed. Intermittent measurements of middle cerebral artery (MCA) velocity via transcranial Doppler ultrasound were also made 2 h before drug administration and continued for 24 h after dosing. The patients were ventilated and sedated with morphine and either midazolam or propofol. There were no behavioral changes during or after administration of the drug, and disorders of perception were reported by only three subjects, several days after relatively low doses; these were transient and were not recalled at later follow-up. We did not detect consistent changes in any of the hemodynamic parameters monitored, up to dosages of 3 mg/kg. After higher doses, some patients showed changes in MABP, ICP, and temperature during the 4 to 8-h period following the first bolus of the drug, with a return toward baseline afterwards. No consistent, serious, adverse events were considered to be due to drug effects, and death, in the one patient who died, was due to the effects of the injury. Our results indicate that CGS 19755 may be given at dosages < or = 3-5 mg/kg with acceptable safety and tolerability in stable, ventilated, and carefully monitored severe head-injured patients.
Effects of (+)-HA-966, CGS-19755, phencyclidine, and dizocilpine on repeated acquisition of response chains in pigeons: systemic manipulation of central glycine sites.[Pubmed:10087045]
J Pharmacol Exp Ther. 1999 Apr;289(1):521-7.
The effects of i.m. injections of (+)-HA-966, a glycine-site antagonist at the N-methyl-D-aspartate (NMDA) subtype of the glutamate receptor, its enantiomer (-)-HA-966, the competitive glutamate antagonist CGS-19755, the uncompetitive glutamate antagonists phencyclidine and dizocilpine, and the micro opioid agonist morphine were evaluated in a repeated acquisition task in pigeons. All of the drugs produced dose-dependent decreases in rates of responding. The NMDA receptor and channel blockers and (+)-HA-966 appeared to have a greater effect on acquisition than did morphine at doses that did not fully suppress responding. The rate suppression and learning impairment produced by a large dose of (+)-HA-966 (100 mg/kg) were completely prevented by coadministration of the glycine-site agonist D-serine (560 mg/kg) but not by its enantiomer, L-serine (1000 mg/kg). D-Serine, however, produced incomplete antagonism of the effects of dizocilpine and phencyclidine and failed to alter those of CGS-19755. These findings provide evidence that reducing the activity of the NMDA subtype of the glutamate receptor through pharmacological action at any of three sites produces similar decrements in acquisition, and those produced through antagonism of the glycine site are differentially sensitive to the glycine-site agonist D-serine.
The NMDA receptor antagonist CGS 19755 disrupts recovery following cerebellar lesions.[Pubmed:16518022]
Restor Neurol Neurosci. 2006;24(1):1-7.
PURPOSE: To test whether activation of NMDA receptors is required for the maintenance of the posture and motor behavior recovered from cerebellar lesions, an NMDA antagonist (CGS 19755) was systemically administered to totally or partially cerebellectomized rats. METHODS: Three groups of animals were tested: rats that had undergone a total cerebellectomy four months before drug administration; rats that had undergone a right hemicerebellectomy four months before drug administration; intact control animals. RESULTS: Under drug action in the control animals the postural pattern was slightly influenced, showing a light worsening, and motor skills requiring coordinated motor performance and subtle balance control were markedly worsened. Conversely, in the lesioned groups the cerebellar symptomatology dramatically worsened, and both groups of animals looked like they had just been operated, exhibiting the whole repertoire of postural and motor behaviors of cerebellar origin. In particular, limb hyperflexion, wide-based locomotion and the tendency to side falls were prevalent in the cerebellectomized animals, while tremor and body tilt were prevalent in the hemicerebellectomized group. CONCLUSION: The reappearance of severe postural and motor symptomatology has to be interpreted as a "decompensation" evoked by the NMDA-receptor antagonist, suggesting the involvement of NMDA receptors in the maintenance of compensation of disturbances of cerebellar origin.
Correlation of CGS 19755 neuroprotection against in vitro excitotoxicity and focal cerebral ischemia.[Pubmed:7673380]
J Cereb Blood Flow Metab. 1995 Sep;15(5):865-76.
The in vivo neuroprotective effect and brain levels of cis-4-phosphonomethyl-2-piperidine carboxylic acid (CGS 19755), a competitive N-methyl-D-aspartate (NMDA) antagonist, were compared with its in vitro neuroprotective effects. The dose-response for in vitro neuroprotection against both NMDA toxicity and combined oxygen-glucose deprivation (OGD) was determined in murine neocortical cultures. Primary cultures of neocortical cells from feta mice were injured by exposure to 500 microM NMDA for 10 min or to OGD for 45 min. The effect of CGS 19755 in both injury paradigms was assessed morphologically and quantitated by determination of lactate dehydrogenase release. Near complete neuroprotection was found at high doses of CGS 19755. The ED50 for protection against NMDA toxicity was 25.4 micro M, and against OGD the ED50 was 15.2 microM. For the in vivo paradigm rabbits underwent 2 h of left internal carotid, anterior cerebral, and middle cerebral artery occlusion followed by 4 h reperfusion; ischemic injury was assessed by magnetic resonance imaging and histopathology. The rabbits were treated with 40 mg/kg i.v. CGS 19755 or saline 10 min after arterial occlusion. CSF and brain levels of CGS 19755 were 12 microM and 5 microM, respectively, at 1 h, 6 microM and 5 microM at 2 h, and 13 microM and 7 microM at 4 h. These levels were neuroprotective in this model, reducing cortical ischemic edema by 48% and ischemic neuronal damage by 76%. These results suggest that a single i.v. dose penetrates the blood-brain barrier, attaining sustained neuroprotective levels that are in the range for in vitro neuroprotection.
Behavioral pharmacological profile of CGS 19755, a competitive antagonist at N-methyl-D-aspartate receptors.[Pubmed:2547931]
J Pharmacol Exp Ther. 1989 Aug;250(2):454-60.
CGS 19755 (cis-4-phosphonomethyl-2-piperidine-carboxylic acid), a competitive antagonist at N-methyl-D-aspartate (NMDA)-preferring receptors, blocked both NMDA-induced convulsions in normal CF1 mice and sound-induced wild running in seizure-prone DBA/2 mice. The ED50 values for CGS 19755 to produce these effects (in the range of 2 mg/kg i.p.) were at least 3-fold lower than those which impaired the traction reflex, an index of motor coordination. When administered p.o. by gavage, CGS 19755 had little or no effect in these test procedures. In an experimental model of anxiety in rats, CGS 19755 significantly increased conflict responding within a relatively narrow dose range (minimum effective dose, 1.73 mg/kg i.p.). At higher doses of CGS 19755, this effect appeared to be obscured by drug-induced reductions in overall responding. Potential muscle relaxant effects were also suggested by the generalization of CGS 19755 to diazepam discriminative stimuli (ED50 = 9.0 mg/kg i.p.) and by impaired rotorod performance (ED50 = 6.2 mg/kg i.p.) in rats. Although some resemblances were apparent between the behavioral effects of CGS 19755 and those of phencyclidine-type drugs, the phencyclidine-like behaviors appeared only at considerably higher doses of CGS 19755 than those associated with anticonflict activity, and only partial generalization of CGS 19755 to dexoxadrol was observed at high doses. CGS 19755 promises to be an important new research tool for investigating the function of brain NMDA receptors.
CGS 19755, a selective and competitive N-methyl-D-aspartate-type excitatory amino acid receptor antagonist.[Pubmed:2899170]
J Pharmacol Exp Ther. 1988 Jul;246(1):65-75.
CGS 19755 (cis-4-phosphonomethyl-2-piperidine carboxylic acid) was found to be a potent, stereospecific inhibitor of N-methyl-D-aspartate (NMDA)-evoked, but not KCl-evoked, [3H] acetylcholine release from slices of the rat striatum. The concentration-response curve to NMDA was shifted to the right by CGS 19755 (pA2 = 5.94), suggesting a competitive interaction with NMDA-type receptors. CGS 19755 inhibited the binding of [3H]-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid to NMDA-type receptors with an IC50 of 50 nM, making it the most potent NMDA-type receptor antagonist reported to date. CGS 19755 failed to interact with 23 other receptor types as assessed by receptor binding, including the quisqualate- and kainate-type excitatory amino acid receptors. In crude P2 fractions, no evidence was obtained to suggest that CGS 19755 is taken up by an active transport system. Furthermore, CGS 19755 failed to affect the uptake of L-[3H]glutamate, or to interact with aconitine-induced inhibition of L-[3H]glutamate uptake, the latter finding suggesting a lack of membrane-stabilizing or local anesthetic properties. CGS 19755 selectively antagonized the excitatory effect of iontophoretically applied NMDA in the red nucleus of the rat without affecting the excitatory effects of quisqualate. CGS 19755 blocked the harmaline-induced increase in cerebellar cyclic GMP levels at a dose of 4 mg/kg i.p. with a duration of action exceeding 2 hr. CGS 19755 inhibited convulsions elicited by maximal electroshock in rat (ED50 = 3.8 mg/kg i.p. 1 hr after administration) and in mouse (ED50 = 2.0 mg/kg i.p. 0.5 hr after administration). Likewise, convulsions elicited by picrotoxin were inhibited by CGS 19755, whereas the compound was relatively weak in protecting against convulsions elicited by pentylenetetrazole or strychnine. CGS 19755 produced retention performance deficits in a dark avoidance task. However, CGS 19755 did not show a unique propensity for learning and memory disruption compared to other anticonvulsants.


