LY2835219CDK4/6 inhibitor,potent and selective CAS# 1231930-82-7 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- LEE011 succinate hydrate
Catalog No.:BCC4103
CAS No.:1374639-79-8
- AMG 925
Catalog No.:BCC5150
CAS No.:1401033-86-0
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- SB1317
Catalog No.:BCC1925
CAS No.:937270-47-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1231930-82-7 | SDF | Download SDF |
| PubChem ID | 71576678 | Appearance | Powder |
| Formula | C28H36F2N8O3S | M.Wt | 602.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ABEMACICLIB; CDK4/6 dual inhibitor | ||
| Solubility | H2O : 125 mg/mL (207.40 mM; Need ultrasonic) DMSO : ≥ 25 mg/mL (41.48 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine;methanesulfonic acid | ||
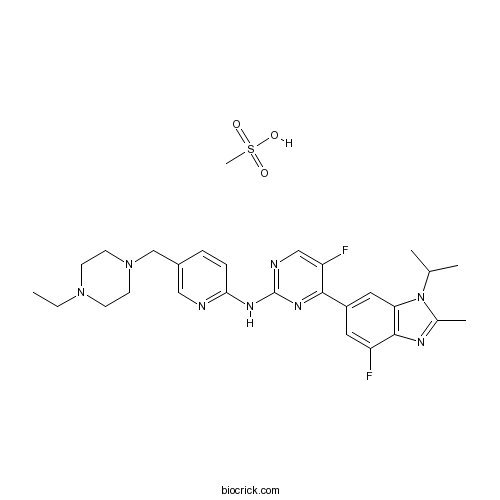
| SMILES | CCN1CCN(CC1)CC2=CN=C(C=C2)NC3=NC=C(C(=N3)C4=CC5=C(C(=C4)F)N=C(N5C(C)C)C)F.CS(=O)(=O)O | ||
| Standard InChIKey | NCJPFQPEVDHJAZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H32F2N8.CH4O3S/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26;1-5(2,3)4/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY2835219 is a potent and selective inhibitor of CDK4 and CDK6 with IC50 value of 2 nM and 10 nM, respectively. | |||||
| Targets | CDK4 | CDK6 | ||||
| IC50 | 2 nM | 10 nM | ||||
| Kinase experiment [1]: | |
| Binding assays | CDK4 and CDK6 activity was determined by radiometric-filter binding assay using a c-terminus fragment of the human Rb protein (containing amino acids 773 to 928) as a substrate. Human CDK4/cyclin D1 and CDK6/cyclin D1 complexes were expressed in insect cells and purified as described. LY2835219 were serially diluted 1:3 in 20 % DMSO to create a 10-point curve at a starting concentration of 20 μM. 20 % DMSO buffer alone was employed as a control; 500 mM EDTA was used to determine the level of background in the absence of enzyme activity. A 4-parameter logistic curve fit was used to generate the IC50 values using ActivityBase software (IDBS). For kinetic analysis, a range of ATP concentrations was used and Ki for both CDK4/cyclin D1 and CDK6/cyclin D1 complexes was determined by fitting to the Michaelis-Menten equation for a competitive inhibitor using GraphPad Prism. |
| Cell experiment [1]: | |
| Cell lines | Colo-205 colorectal cells, MDA-MB-361 and MCF10A breast cancer cell lines, MV4-11 AML cells. |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 24 h |
| Applications | LY2835219 is a selective and orally available dual cyclin-dependent kinases 4/6 (CDK4/6) inhibitor. LY2835219 (6000 nM) inhibits Rb phosphorylation with IC50 value of 120 nM and a corresponding arrest of cells in G1 (2 N DNA content) with EC50 value of 72 nM. |
| Animal experiment [1]: | |
| Animal models | Mice bearing colo-205 xenograft tumors. |
| Dosage form | 12.5 mg/kg, 25 to 100mg/kg |
| Preparation method | Formulated in 1 % hydroxyethyl cellulose + 0.1 % antifoam in 25 mM PB pH 2 and administered orally by gavage (final volume 0.2 mL). |
| Application | LY2835219 mediates CDK4/6 inhibition, cell-cycle arrest and tumor growth inhibition (TGI) in colo-205 and inhibits Rb phosphorylation by CDK4/6. LY2835219 significantly inhibits tumor growth, doses up to 100 mg/kg are well tolerated with no loss of body weight or other signs of toxicity during or after treatment. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1]. Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs, 2014, 32(5): 825-837. | |

LY2835219 Dilution Calculator

LY2835219 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6592 mL | 8.296 mL | 16.592 mL | 33.184 mL | 41.48 mL |
| 5 mM | 0.3318 mL | 1.6592 mL | 3.3184 mL | 6.6368 mL | 8.296 mL |
| 10 mM | 0.1659 mL | 0.8296 mL | 1.6592 mL | 3.3184 mL | 4.148 mL |
| 50 mM | 0.0332 mL | 0.1659 mL | 0.3318 mL | 0.6637 mL | 0.8296 mL |
| 100 mM | 0.0166 mL | 0.083 mL | 0.1659 mL | 0.3318 mL | 0.4148 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2835219 is a selective and orally available dual cyclin-dependent kinases 4/6 (CDK4/6) inhibitor that potently inhibits the activities of CDK4 and CDK6 with the half maximal inhibition concentration IC50 values of 2 nM and 10 nM respectively [1].
LY2835219 has also been found to inhibit Rb phosphorylation both in vivo and in vitro leading to specific cell arrest at G1 phase as well as the inhibition of tumor growth [1].
Since the blood brain barrier (BBB) is a major obstacle for the effective treatment of primary brain tumors and brain metastases, LY2835219, which is able to cross the BBB, has the potential to inhibit intracranial tumor growth alone or in combination with other agents [1].
References:
[1] Concepcion Sanchez-Martinez, Lawrence M. Gelbert, Harlan Shannon, Alfonso De Dios, Brian A. Staton, Rose T. Ajamie, Geri Sawada, Graham N. Wishart and Thomas J. Raub. LY2835219, a potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) that crosses the blood-brain barrier and demonstrates in vivo activity against intracranial human brain tumor xenografts [abstract]. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2011 Nov 12-16; San Francisco, CA. Philadelphia (PA): AACR; Mol Cancer Ther 2011;10(11 Suppl):Abstract nr B234.
- LY2835219 free base
Catalog No.:BCC1722
CAS No.:1231929-97-7
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Azelaic Acid
Catalog No.:BCC8300
CAS No.:123-99-9
- D-erythro-Sphingosine (synthetic)
Catalog No.:BCC6729
CAS No.:123-78-4
- Anisic aldehyde
Catalog No.:BCN2618
CAS No.:123-11-5
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
The CDK4/6 inhibitor LY2835219 has potent activity in combination with mTOR inhibitor in head and neck squamous cell carcinoma.[Pubmed:26909611]
Oncotarget. 2016 Mar 22;7(12):14803-13.
Deletion of CDKN2A (p16) or amplification of CCND1 (cyclin D1) occurs commonly in head and neck squamous cell carcinoma (HNSCC) and induces sustained cyclin-dependent kinase (CDK) 4/6 activation. Here, we report the antiproliferative activity of LY2835219, a selective CDK4/6 inhibitor through inhibition of CDK4/6-dependent Ser780 phosphorylation in retinoblastoma (RB) and induction of cell cycle arrest in HNSCC cells. In addition, we demonstrated the antitumor effects of HNSCC xenografts to LY2835219 in vivo. Given the limited effect in HNSCC as a single-agent treatment with LY2835219, a combinational strategy is required to enhance antitumor activity. At the molecular level, we found that LY2835219 inhibited activation of AKT and ERK, but not mTOR. The combination of LY2835219 with mTOR inhibitor was found to be more effective than either drug alone in vitro and in vivo. Taken together, our findings suggest that a combinational treatment with LY2835219 and mTOR inhibitor is a promising therapeutic approach for HNSCC.
Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine.[Pubmed:24919854]
Invest New Drugs. 2014 Oct;32(5):825-37.
The G1 restriction point is critical for regulating the cell cycle and is controlled by the Rb pathway (CDK4/6-cyclin D1-Rb-p16/ink4a). This pathway is important because of its inactivation in a majority of human tumors. Transition through the restriction point requires phosphorylation of retinoblastoma protein (Rb) by CDK4/6, which are highly validated cancer drug targets. We present the identification and characterization of a potent CDK4/6 inhibitor, LY2835219. LY2835219 inhibits CDK4 and CDK6 with low nanomolar potency, inhibits Rb phosphorylation resulting in a G1 arrest and inhibition of proliferation, and its activity is specific for Rb-proficient cells. In vivo target inhibition studies show LY2835219 is a potent inhibitor of Rb phosphorylation, induces a complete cell cycle arrest and suppresses expression of several Rb-E2F-regulated proteins 24 hours after a single dose. Oral administration of LY2835219 inhibits tumor growth in human tumor xenografts representing different histologies in tumor-bearing mice. LY2835219 is effective and well tolerated when administered up to 56 days in immunodeficient mice without significant loss of body weight or tumor outgrowth. In calu-6 xenografts, LY2835219 in combination with gemcitabine enhanced in vivo antitumor activity without a G1 cell cycle arrest, but was associated with a reduction of ribonucleotide reductase expression. These results suggest LY2835219 may be used alone or in combination with standard-of-care cytotoxic therapy. In summary, we have identified a potent, orally active small-molecule inhibitor of CDK4/6 that is active in xenograft tumors. LY2835219 is currently in clinical development.
Effect of abemaciclib (LY2835219) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo.[Pubmed:27816545]
Biochem Pharmacol. 2017 Jan 15;124:29-42.
Multidrug resistance (MDR) is the major obstacle of the success in cancer chemotherapy. The overexpression of ATP-binding cassette (ABC) transporters, particularly ABCB1 and ABCG2, play a significant role in mediating MDR by pumping anticancer drugs out of cancer cells. Abemaciclib (LY2835219) is an orally bioavailable CDK4/6 inhibitor under phase III clinical trials. Here, we found that LY2835219 remarkably enhanced the efficacy of chemotherapeutic drugs in ABCB1 or ABCG2 over-expressing cancer cells in vitro and in vivo. Furthermore, LY2835219 significantly increased the intracellular accumulation of doxorubicin (DOX) and rhodamine 123 (Rho 123) by inhibiting ABCB1 or ABCG2-mediated drug efflux in the transporters-overexpressing cells. Mechanistically, LY2835219 is likely a competitive inhibitor of ABCB1 and ABCG2 for its competition with [125I]-iodoarylazidoprazosin for photo affinity labeling of the transporters. On the other hand, at the transporters-inhibiting concentrations, LY2835219 did not alter the expression level of ABCB1 and ABCG2, and the phosphorylation status of retinoblastoma (Rb) pathway in both parental and their resistant cells. In conclusion, these findings revealed a novel role of LY2835219 in reversing ABCB1 or ABCG2-mediated MDR, which may be benefit to the patients with MDR cancer for combinational therapy.
The CDK4/6 inhibitor LY2835219 overcomes vemurafenib resistance resulting from MAPK reactivation and cyclin D1 upregulation.[Pubmed:25122067]
Mol Cancer Ther. 2014 Oct;13(10):2253-63.
B-RAF selective inhibitors, including vemurafenib, were recently developed as effective therapies for melanoma patients with B-RAF V600E mutation. However, most patients treated with vemurafenib eventually develop resistance largely due to reactivation of MAPK signaling. Inhibitors of MAPK signaling, including MEK1/2 inhibitor trametinib, failed to show significant clinical benefit in patients with acquired resistance to vemurafenib. Here, we describe that cell lines with acquired resistance to vemurafenib show reactivation of MAPK signaling and upregulation of cyclin D1 and are sensitive to inhibition of LY2835219, a selective inhibitor of cyclin-dependent kinase (CDK) 4/6. LY2835219 was demonstrated to inhibit growth of melanoma A375 tumor xenografts and delay tumor recurrence in combination with vemurafenib. Furthermore, we developed an in vivo vemurafenib-resistant model by continuous administration of vemurafenib in A375 xenografts. Consistently, we found that MAPK is reactivated and cyclin D1 is elevated in vemurafenib-resistant tumors, as well as in the resistant cell lines derived from these tumors. Importantly, LY2835219 exhibited tumor growth regression in a vemurafenib-resistant model. Mechanistic analysis revealed that LY2835219 induced apoptotic cell death in a concentration-dependent manner in vemurafenib-resistant cells whereas it primarily mediated cell-cycle G1 arrest in the parental cells. Similarly, RNAi-mediated knockdown of cyclin D1 induced significantly higher rate of apoptosis in the resistant cells than in parental cells, suggesting that elevated cyclin D1 activity is important for the survival of vemurafenib-resistant cells. Altogether, we propose that targeting cyclin D1-CDK4/6 signaling by LY2835219 is an effective strategy to overcome MAPK-mediated resistance to B-RAF inhibitors in B-RAF V600E melanoma.


