Anisic aldehydeCAS# 123-11-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123-11-5 | SDF | Download SDF |
| PubChem ID | 31244 | Appearance | Oil |
| Formula | C8H8O2 | M.Wt | 136.14 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
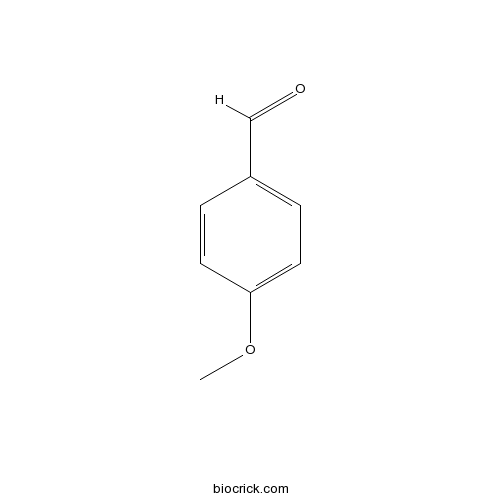
| Chemical Name | 4-methoxybenzaldehyde | ||
| SMILES | COC1=CC=C(C=C1)C=O | ||
| Standard InChIKey | ZRSNZINYAWTAHE-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anisic aldehyde is a safe food additive for human, it has significant antifungal activity against Candida, including azole-resistant strains. |
| Targets | Antifection |
| In vitro | Determination on lead and arsenic in anisic aldehyde[Reference: WebLink]《Journal of Central South University of Forestry & Technology》 2013-03
|

Anisic aldehyde Dilution Calculator

Anisic aldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3454 mL | 36.7269 mL | 73.4538 mL | 146.9076 mL | 183.6345 mL |
| 5 mM | 1.4691 mL | 7.3454 mL | 14.6908 mL | 29.3815 mL | 36.7269 mL |
| 10 mM | 0.7345 mL | 3.6727 mL | 7.3454 mL | 14.6908 mL | 18.3634 mL |
| 50 mM | 0.1469 mL | 0.7345 mL | 1.4691 mL | 2.9382 mL | 3.6727 mL |
| 100 mM | 0.0735 mL | 0.3673 mL | 0.7345 mL | 1.4691 mL | 1.8363 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- Fmoc-Hyp(tBu)-OH
Catalog No.:BCC3256
CAS No.:122996-47-8
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- HA 130
Catalog No.:BCC7884
CAS No.:1229652-21-4
- 6'-O-Galloyl paeoniflorin
Catalog No.:BCN2941
CAS No.:122965-41-7
- URMC-099
Catalog No.:BCC5563
CAS No.:1229582-33-5
- XE 991 dihydrochloride
Catalog No.:BCC7232
CAS No.:122955-13-9
- N-(3-Methoxybenzyl)palmitamide
Catalog No.:BCN8086
CAS No.:847361-96-0
- Fmoc-L-Beta-Homoproline
Catalog No.:BCN8087
CAS No.:193693-60-6
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
- C 21
Catalog No.:BCC8013
CAS No.:1229236-78-5
- GS-9973
Catalog No.:BCC5278
CAS No.:1229208-44-9
- D-erythro-Sphingosine (synthetic)
Catalog No.:BCC6729
CAS No.:123-78-4
- Azelaic Acid
Catalog No.:BCC8300
CAS No.:123-99-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
- LY2835219 free base
Catalog No.:BCC1722
CAS No.:1231929-97-7
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
Identification, expression profiling and fluorescence-based binding assays of a chemosensory protein gene from the Western flower thrips, Frankliniella occidentalis.[Pubmed:25635391]
PLoS One. 2015 Jan 30;10(1):e0117726.
Using RT-PCR and RACE-PCR strategies, we cloned and identified a new chemosensory protein (FoccCSP) from the Western flower thrips, Frankliniella occidentalis, a species for which no chemosensory protein (CSP) has yet been identified. The FoccCSP gene contains a 387 bp open-reading frame encoding a putative protein of 128 amino acids with a molecular weight of 14.51 kDa and an isoelectric point of 5.41. The deduced amino acid sequence contains a putative signal peptide of 19 amino acid residues at the N-terminus, as well as the typical four-cysteine signature found in other insect CSPs. As FoccCSP is from a different order of insect than other known CSPs, the GenBank FoccCSP homolog showed only 31-50% sequence identity with them. A neighbor-joining tree was constructed and revealed that FoccCSP is in a group with CSPs from Homopteran insects (e.g., AgosCSP4, AgosCSP10, ApisCSP, and NlugCSP9), suggesting that these genes likely developed from a common ancestral gene. The FoccCSP gene expression profile of different tissues and development stages was measured by quantitative real-time PCR. The results of this analysis revealed this gene is predominantly expressed in the antennae and also highly expressed in the first instar nymph, suggesting a function for FoccCSP in olfactory reception and in particular life activities during the first instar nymph stage. We expressed recombinant FoccCSP protein in a prokaryotic expression system and purified FoccCSP protein by affinity chromatography using a Ni-NTA-Sepharose column. Using N-phenyl-1-naphthylamine (1-NPN) as a fluorescent probe in fluorescence-based competitive binding assay, we determined the binding affinities of 19 volatile substances for FoccCSP protein. This analysis revealed that Anisic aldehyde, geraniol and methyl salicylate have high binding affinities for FoccCSP, with KD values of 10.50, 15.35 and 35.24 muM, respectively. Thus, our study indicates that FoccCSP may play an important role in regulating the development of the first instar nymph and mediate F. occidentalis host recognition.
Interesting anticandidal effects of anisic aldehydes on growth and proton-pumping-ATPase-targeted activity.[Pubmed:21669279]
Microb Pathog. 2011 Oct;51(4):277-84.
Attention has been drawn to evaluate the antifungal activity of p-anisaldehyde (1), o-anisaldehyde (2) and m-anisaldehyde (3). To put forward this approach, antifungal activity has been assessed in thirty six fluconazole-sensitive and eleven fluconazole-resistant Candida isolates. Growth and sensitivity of the organisms were significantly effected by test compounds at different concentrations. The rapid irreversible action of compound-1, compound-2 and compound-3 on fungal cells suggested a membrane-located target for their action. We investigated their effect on H(+) ATPase mediated H(+)-pumping by various Candida species. All the compounds inhibit H(+)- ATPase activity at their respective MIC(90) values. Inhibition of H(+) ATPase leads to intracellular acidification and cell death. Scanning electron microscopy analysis revealed deep wrinkles, deformity and flowed content. Furthermore, it was also observed that position of methoxy group attached to the benzene ring decides antifungal activity of the compound. The present study indicates that compound-1, compound-2 and compound-3 have significant antifungal activity against Candida, including azole-resistant strains, advocating further investigation for clinical applications in the treatment of fungal infections.


