Azelaic AcidCAS# 123-99-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123-99-9 | SDF | Download SDF |
| PubChem ID | 2266 | Appearance | Powder |
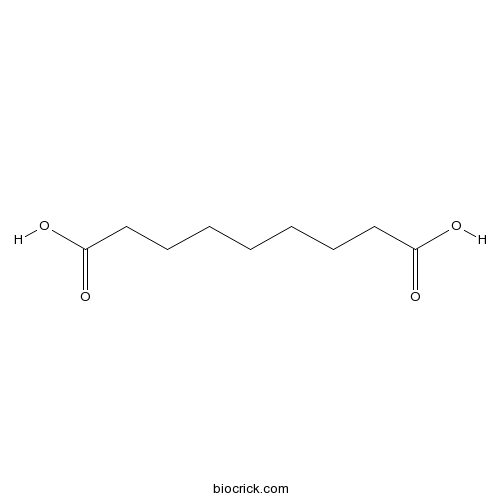
| Formula | C9H16O4 | M.Wt | 188 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Nonanedioic acid | ||
| Solubility | DMSO : ≥ 100 mg/mL (531.29 mM) H2O : 2 mg/mL (10.63 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | nonanedioic acid | ||
| SMILES | C(CCCC(=O)O)CCCC(=O)O | ||
| Standard InChIKey | BDJRBEYXGGNYIS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H16O4/c10-8(11)6-4-2-1-3-5-7-9(12)13/h1-7H2,(H,10,11)(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Azelaic Acid Dilution Calculator

Azelaic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3191 mL | 26.5957 mL | 53.1915 mL | 106.383 mL | 132.9787 mL |
| 5 mM | 1.0638 mL | 5.3191 mL | 10.6383 mL | 21.2766 mL | 26.5957 mL |
| 10 mM | 0.5319 mL | 2.6596 mL | 5.3191 mL | 10.6383 mL | 13.2979 mL |
| 50 mM | 0.1064 mL | 0.5319 mL | 1.0638 mL | 2.1277 mL | 2.6596 mL |
| 100 mM | 0.0532 mL | 0.266 mL | 0.5319 mL | 1.0638 mL | 1.3298 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Azelaic acid is an organic compound produced by the ozonolysis of oleic acid; component of a number of hair and skin conditioners.
References:
[1]. Jung HW, et al. Priming in systemic plant immunity. Science. 2009 Apr 3;324(5923):89-91.
[2]. Liu RH, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006 Aug;142(8):1047-52.
- D-erythro-Sphingosine (synthetic)
Catalog No.:BCC6729
CAS No.:123-78-4
- Anisic aldehyde
Catalog No.:BCN2618
CAS No.:123-11-5
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- Fmoc-Hyp(tBu)-OH
Catalog No.:BCC3256
CAS No.:122996-47-8
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- HA 130
Catalog No.:BCC7884
CAS No.:1229652-21-4
- 6'-O-Galloyl paeoniflorin
Catalog No.:BCN2941
CAS No.:122965-41-7
- URMC-099
Catalog No.:BCC5563
CAS No.:1229582-33-5
- XE 991 dihydrochloride
Catalog No.:BCC7232
CAS No.:122955-13-9
- N-(3-Methoxybenzyl)palmitamide
Catalog No.:BCN8086
CAS No.:847361-96-0
- Fmoc-L-Beta-Homoproline
Catalog No.:BCN8087
CAS No.:193693-60-6
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
- LY2835219 free base
Catalog No.:BCC1722
CAS No.:1231929-97-7
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Facial Acne Vulgaris.[Pubmed:29879251]
J Drugs Dermatol. 2018 Jun 1;17(6):641-645.
BACKGROUND: Azelaic Acid demonstrates anti-inflammatory, anti-oxidative, anti-comedogenic, and anti-microbial effects. Azelaic Acid 20% cream is currently approved for the treatment of acne vulgaris, and Azelaic Acid 15% foam has recently been approved for rosacea. Given the favorable tolerability profile of foam preparations, it is reasonable to assume that Azelaic Acid 15% foam could serve as a viable treatment option for facial acne. OBJECTIVE: To examine the efficacy and safety of Azelaic Acid 15% foam in the treatment of moderate-to-severe facial acne Methods: Twenty subjects with moderate-to-severe facial acne vulgaris were enrolled in this two-center, open-label pilot study. All study subjects were treated with Azelaic Acid 15% foam for 16 weeks. Efficacy analyses were based on the change in facial investigator global assessment (FIGA) and changes in total, inflammatory, non-inflammatory lesion counts between baseline and week 16. RESULTS: There was a significant reduction in FIGA scores from baseline to week 16 (p = .0004), with 84% of subjects experiencing at least a 1 grade improvement, and 63% of subjects achieving a final grade of Clear or Almost Clear. All subjects experienced reductions in inflammatory and total lesion counts by week 16, and 89% of subjects experienced reductions in non-inflammatory lesions. Azelaic Acid 15% foam was well tolerated, with almost all instances of erythema, dryness, peeling, oiliness, pruritus, and burning being of mild or trace degree, and most adverse effects resolving by the end of the study. CONCLUSION: Azelaic Acid 15% foam is effective and safe in the treatment of facial acne vulgaris. Given the convenience of foam vehicles, Azelaic Acid 15% foam should be considered as a viable treatment option for this condition. J Drugs Dermatol. 2018;17(6):641-645.


