LY2835219 free baseCDK inhibitor CAS# 1231929-97-7 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1231929-97-7 | SDF | Download SDF |
| PubChem ID | 46220502 | Appearance | Powder |
| Formula | C27H32F2N8 | M.Wt | 506.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Abemaciclib; CDK4/6 dual inhibitor | ||
| Solubility | DMSO : 5 mg/mL (9.87 mM; Need ultrasonic) | ||
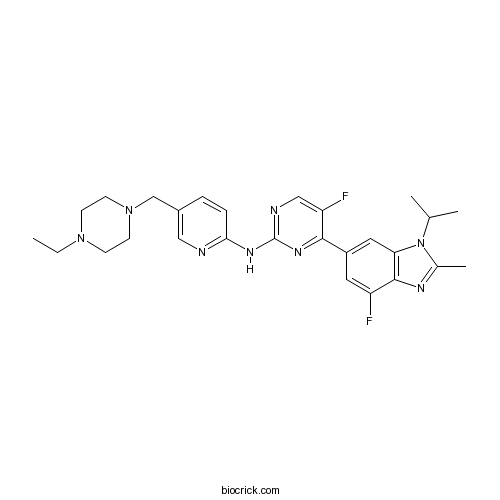
| Chemical Name | N-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine | ||
| SMILES | CCN1CCN(CC1)CC2=CN=C(C=C2)NC3=NC=C(C(=N3)C4=CC5=C(C(=C4)F)N=C(N5C(C)C)C)F | ||
| Standard InChIKey | UZWDCWONPYILKI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY2835219 free base is a selective CDK4/6 inhibitor with IC50 values of 2 nM and 10 nM for CDK4 and CDK6, respectively.In Vitro:LY2835219 reduces cell viability with the IC50 values ranging from 0.5 μM to 0.7 μM, inhibits Akt and ERK signaling but not mTOR activation at head and neck squamous cell carcinoma (HNSCC) cells[1]. LY2835219 shows inhibition on A375R1-4, M14R, and SH4R with EC50 values ranging from 0.3 to 0.6 μM; LY2835219 inhibits the proliferation of the parental A375 and resistant A375RV1 and A375RV2 cells with similar potencies with IC50 values of 395, 260, and 463 nM, respectively[2]. LY2835219 inhibits CDK4 and CDK6 with low nanomolar potency, inhibits Rb phosphorylation resulting in a G1 arrest and inhibition of proliferation, and its activity is specific for Rb-proficient cells[3].In Vivo:LY2835219 (45 mg/kg, p.o.) in combination with everolimus causes a cooperative antitumor effect in HNSCC xenograft tumor[1]. LY2835219 (45 or 90 mg/kg, p.o.) shows significant tumor growth inhibition in an A375 xenograft model[2]. References: | |||||

LY2835219 free base Dilution Calculator

LY2835219 free base Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.974 mL | 9.8699 mL | 19.7398 mL | 39.4797 mL | 49.3496 mL |
| 5 mM | 0.3948 mL | 1.974 mL | 3.948 mL | 7.8959 mL | 9.8699 mL |
| 10 mM | 0.1974 mL | 0.987 mL | 1.974 mL | 3.948 mL | 4.935 mL |
| 50 mM | 0.0395 mL | 0.1974 mL | 0.3948 mL | 0.7896 mL | 0.987 mL |
| 100 mM | 0.0197 mL | 0.0987 mL | 0.1974 mL | 0.3948 mL | 0.4935 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2835219 is an orally available cyclin-dependent kinase (CDK) inhibitor that targets the CDK4 (cyclin D1) and CDK6 (cyclin D3) cell cycle pathway, with potential antineoplastic activity. CDK4/6 dual inhibitor LY2835219 specifically inhibits CDK4 and 6, thereby inhibiting retinoblastoma (Rb) protein phosphorylation in early G1. Inhibition of Rb phosphorylation prevents CDK-mediated G1-S phase transition, thereby arresting the cell cycle in the G1 phase, suppressing DNA synthesis and inhibiting cancer cell growth. Overexpression of the serine/threonine kinases CDK4/6, as seen in certain types of cancer, causes cell cycle deregulation.
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Azelaic Acid
Catalog No.:BCC8300
CAS No.:123-99-9
- D-erythro-Sphingosine (synthetic)
Catalog No.:BCC6729
CAS No.:123-78-4
- Anisic aldehyde
Catalog No.:BCN2618
CAS No.:123-11-5
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- Fmoc-Hyp(tBu)-OH
Catalog No.:BCC3256
CAS No.:122996-47-8
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence.[Pubmed:27748766]
Oncogene. 2017 Apr 20;36(16):2255-2264.
Dysregulated activation of the CDK4/6 kinases is a hallmark of most mammary-derived carcinomas. ATP-competitive inhibitors against this complex have been recently advanced in the clinic and have shown significant activity, particularly against tumors driven by the estrogen receptor (ER). However, resistance to these compounds has begun to emerge often months to years after their initiation. We investigated potential mechanisms of resistance using cell line models that are highly sensitive to this class of drugs. After prolonged exposure to the selective and potent CDK4/6 inhibitor LY2835219, clones emerged and several were found to harbor amplification of the CDK6 kinase. Amplification of CDK6 resulted in a marked increase in CDK6 expression and reduced response of the CDK4/6 target, phospho-Rb (pRb), to CDK4/6 inhibitors. Knockdown of CDK6 restored drug sensitivity, while enforced overexpression of CDK6 was sufficient to mediate drug resistance. Not only did CDK6 overexpression mediate resistance to CDK4/6 inhibitors but it also led to reduced expression of the ER and progesterone receptor (PR), and diminished responsiveness to ER antagonism. The reduced ER/PR expression after CDK4/6 inhibitor resistance was additionally observed in tumor biopsy specimens from patients treated with these drugs. Alternative mechanisms of resistance to CDK4/6 inhibitors such as loss of pRb and cyclin E1 overexpression also exhibited decreased hormone responsiveness, suggesting that the clinical paradigm of sequential endocrine-based therapy may be ineffective in some settings of acquired CDK4/6 resistance.
The CDK4/6 inhibitor LY2835219 overcomes vemurafenib resistance resulting from MAPK reactivation and cyclin D1 upregulation.[Pubmed:25122067]
Mol Cancer Ther. 2014 Oct;13(10):2253-63.
B-RAF selective inhibitors, including vemurafenib, were recently developed as effective therapies for melanoma patients with B-RAF V600E mutation. However, most patients treated with vemurafenib eventually develop resistance largely due to reactivation of MAPK signaling. Inhibitors of MAPK signaling, including MEK1/2 inhibitor trametinib, failed to show significant clinical benefit in patients with acquired resistance to vemurafenib. Here, we describe that cell lines with acquired resistance to vemurafenib show reactivation of MAPK signaling and upregulation of cyclin D1 and are sensitive to inhibition of LY2835219, a selective inhibitor of cyclin-dependent kinase (CDK) 4/6. LY2835219 was demonstrated to inhibit growth of melanoma A375 tumor xenografts and delay tumor recurrence in combination with vemurafenib. Furthermore, we developed an in vivo vemurafenib-resistant model by continuous administration of vemurafenib in A375 xenografts. Consistently, we found that MAPK is reactivated and cyclin D1 is elevated in vemurafenib-resistant tumors, as well as in the resistant cell lines derived from these tumors. Importantly, LY2835219 exhibited tumor growth regression in a vemurafenib-resistant model. Mechanistic analysis revealed that LY2835219 induced apoptotic cell death in a concentration-dependent manner in vemurafenib-resistant cells whereas it primarily mediated cell-cycle G1 arrest in the parental cells. Similarly, RNAi-mediated knockdown of cyclin D1 induced significantly higher rate of apoptosis in the resistant cells than in parental cells, suggesting that elevated cyclin D1 activity is important for the survival of vemurafenib-resistant cells. Altogether, we propose that targeting cyclin D1-CDK4/6 signaling by LY2835219 is an effective strategy to overcome MAPK-mediated resistance to B-RAF inhibitors in B-RAF V600E melanoma.
CDK4/6 Inhibition Controls Proliferation of Bladder Cancer and Transcription of RB1.[Pubmed:26318986]
J Urol. 2016 Mar;195(3):771-9.
PURPOSE: The retinoblastoma signaling network is frequently altered in advanced bladder cancer. We investigated the potential of CDK4/6 as a therapeutic target and determined biomarkers for patient stratification. MATERIALS AND METHODS: Genetic alterations were analyzed using public databases, including TCGA (The Cancer Genome Atlas), COSMIC (Catalogue of Somatic Mutations in Cancer) and CCLE (Cancer Cell Line Encyclopedia). Effects of the CDK4/6-inhibitor PD-0332991 or LY2835219 were examined in 10 bladder cancer cell lines by immunoblot, cell viability, apoptosis and cell cycle progression. Efficacy of the PD-0332991 and cisplatin combination was analyzed using the combination index. Gene expression level was determined by quantitative polymerase chain reaction. Cytomegalovirus promoter regulated recombinant retinoblastoma was used for reconstitution. Three-dimensional xenografts were grown on chicken chorioallantoic membrane and analyzed by measuring tumor weight and immunohistochemical expression of total retinoblastoma and Ki-67. RESULTS: PD-0332991 treatment decreased the proliferation of retinoblastoma positive bladder cancer cell lines and was synergistic in combination with cisplatin. PD-0332991 or LY2835219 treatment decreased the phosphorylation, total protein and transcript level of retinoblastoma. Treatment resulted in a decrease in E2F target gene expression (CCNA2 and CCNE2) and cell cycle progression from G0/G1 to the S-phase but did not affect apoptosis. In retinoblastoma negative cells reconstituted with recombinant retinoblastoma PD-0332991 affected only phosphorylation and not the total retinoblastoma level. These cells remained resistant to treatment. In 3-dimensional retinoblastoma xenografts, treatment resulted in reduced tumor weight and decreased expression of total retinoblastoma and Ki-67. CONCLUSIONS: We provide preclinical evidence that CDK4/6 inhibition is a potential therapeutic strategy for retinoblastoma positive bladder cancer that probably acts by negatively regulating retinoblastoma transcription.
The CDK4/6 inhibitor LY2835219 has potent activity in combination with mTOR inhibitor in head and neck squamous cell carcinoma.[Pubmed:26909611]
Oncotarget. 2016 Mar 22;7(12):14803-13.
Deletion of CDKN2A (p16) or amplification of CCND1 (cyclin D1) occurs commonly in head and neck squamous cell carcinoma (HNSCC) and induces sustained cyclin-dependent kinase (CDK) 4/6 activation. Here, we report the antiproliferative activity of LY2835219, a selective CDK4/6 inhibitor through inhibition of CDK4/6-dependent Ser780 phosphorylation in retinoblastoma (RB) and induction of cell cycle arrest in HNSCC cells. In addition, we demonstrated the antitumor effects of HNSCC xenografts to LY2835219 in vivo. Given the limited effect in HNSCC as a single-agent treatment with LY2835219, a combinational strategy is required to enhance antitumor activity. At the molecular level, we found that LY2835219 inhibited activation of AKT and ERK, but not mTOR. The combination of LY2835219 with mTOR inhibitor was found to be more effective than either drug alone in vitro and in vivo. Taken together, our findings suggest that a combinational treatment with LY2835219 and mTOR inhibitor is a promising therapeutic approach for HNSCC.


