kb NB 142-70Inhibitor of protein kinase D,selective CAS# 1233533-04-4 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1233533-04-4 | SDF | Download SDF |
| PubChem ID | 45258277 | Appearance | Powder |
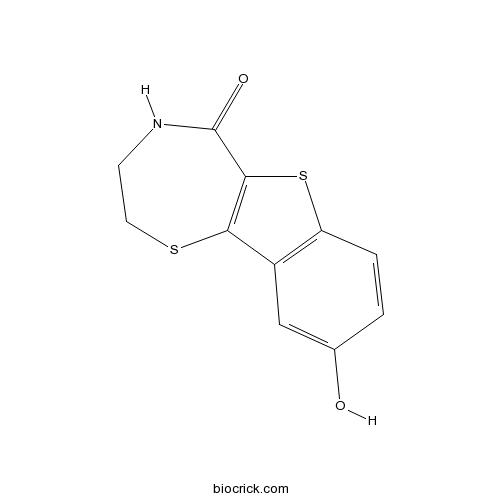
| Formula | C11H9NO2S2 | M.Wt | 251.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 33 mg/mL (131.31 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 9-hydroxy-3,4-dihydro-2H-[1]benzothiolo[2,3-f][1,4]thiazepin-5-one | ||
| SMILES | C1CSC2=C(C(=O)N1)SC3=C2C=C(C=C3)O | ||
| Standard InChIKey | DHUAGGSHTKPOHU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H9NO2S2/c13-6-1-2-8-7(5-6)9-10(16-8)11(14)12-3-4-15-9/h1-2,5,13H,3-4H2,(H,12,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective protein kinase D (PKD) inhibitor (IC50 values are 28.3, 58.7 and 53.2 nM for PKD1, 2 and 3 respectively). More potent analog of CID 755673 with 7-fold greater inhibition. Inhibits prostrate cancer cell migration and invasion and reduces wound healing in vitro; displays prominent cytotoxic and anti-proliferative effects. |

kb NB 142-70 Dilution Calculator

kb NB 142-70 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.979 mL | 19.895 mL | 39.7899 mL | 79.5798 mL | 99.4748 mL |
| 5 mM | 0.7958 mL | 3.979 mL | 7.958 mL | 15.916 mL | 19.895 mL |
| 10 mM | 0.3979 mL | 1.9895 mL | 3.979 mL | 7.958 mL | 9.9475 mL |
| 50 mM | 0.0796 mL | 0.3979 mL | 0.7958 mL | 1.5916 mL | 1.9895 mL |
| 100 mM | 0.0398 mL | 0.1989 mL | 0.3979 mL | 0.7958 mL | 0.9947 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
kb NB 142-70 is a selective protein kinase D (PKD) inhibitor (IC50 values are 28.3, 58.7 and 53.2 nM for PKD1, 2 and 3 respectively). Kb NB 142-70 inhibits prostate cancer cell migration and invasion and reduces wound healing in vitro. Kb NB 142-70 displays prominent cytotoxic and anti-proliferative effects.
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- LY2835219 free base
Catalog No.:BCC1722
CAS No.:1231929-97-7
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
PKD1 is downregulated in non-small cell lung cancer and mediates the feedback inhibition of mTORC1-S6K1 axis in response to phorbol ester.[Pubmed:25578563]
Int J Biochem Cell Biol. 2015 Mar;60:34-42.
Protein kinase D1 (PKD1) is increasingly implicated in multiple biological and molecular events that regulate the proliferation or invasiveness in several cancers. However, little is known about the expression and functions of PKD1 in non-small cell lung cancer (NSCLC). In the present study, 34 pairs of human NSCLC and matched normal bronchiolar epitheliums were enrolled and evaluated for PKD1 expression by quantitative real-time PCR. We showed that PKD1 was downregulated in 26 of 34 cancer tissues in comparison with matched normal epitheliums. Moreover, patients with venous invasion or lymph node metastasis showed significant lower expression of PKD1. Exposure of NSCLC A549 and H520 cells to the PKD family inhibitor kb NB 142-70(Kb), at concentrations that inhibited PKD1 activation, strikingly potentiated S6K1 phosphorylation at Thr(389) and S6 phosphorylation at Ser(235/236) in response to phorbol ester (PMA). Knockdown of PKD1 with siRNAs strikingly enhanced S6K1 phosphorylation whereas constitutively active PKD1 resulted in the S6K1 activity inhibition. Furthermore, the PI3K inhibitors LY294002, BKM120 and MEK inhibitors U0126, PD0325901 blocked the enhanced S6K1 activity induced by Kb. Collectively, our results identify decreased expression of the PKD1 as a marker for NSCLC and the loss of PKD1 expression increases the malignant potential of NSCLC cells. This may be due to the function of PKD1 as a negative regulator of mTORC1-S6K1. Our results suggest that re-expression or activation of PKD1 might serve as a potential therapeutic target for NSCLC treatment.
Insulin Receptor and GPCR Crosstalk Stimulates YAP via PI3K and PKD in Pancreatic Cancer Cells.[Pubmed:28360038]
Mol Cancer Res. 2017 Jul;15(7):929-941.
We examined the impact of crosstalk between the insulin receptor and G protein-coupled receptor (GPCR) signaling pathways on the regulation of Yes-associated protein (YAP) localization, phosphorylation, and transcriptional activity in the context of human pancreatic ductal adenocarcinoma (PDAC). Stimulation of PANC-1 or MiaPaCa-2 cells with insulin and neurotensin, a potent mitogenic combination of agonists for these cells, promoted striking YAP nuclear localization and decreased YAP phosphorylation at Ser(127) and Ser(397) Challenging PDAC cells with either insulin or neurotensin alone modestly induced the expression of YAP/TEAD-regulated genes, including connective tissue growth factor (CTGF), cysteine-rich angiogenic inducer 61 (CYR61), and CXCL5, whereas the combination of neurotensin and insulin induced a marked increase in the level of expression of these genes. In addition, siRNA-mediated knockdown of YAP/TAZ prevented the increase in the expression of these genes. A small-molecule inhibitor (A66), selective for the p110alpha subunit of PI3K, abrogated the increase in phosphatidylinositol 3,4,5-trisphosphate production and the expression of CTGF, CYR61, and CXCL5 induced by neurotensin and insulin. Furthermore, treatment of PDAC cells with protein kinase D (PKD) family inhibitors (CRT0066101 or kb NB 142-70) or with siRNAs targeting the PKD family prevented the increase of CTGF, CYR61, and CXCL5 mRNA levels in response to insulin and neurotensin stimulation. Thus, PI3K and PKD mediate YAP activation in response to insulin and neurotensin in pancreatic cancer cells.Implications: Inhibitors of PI3K or PKD disrupt crosstalk between insulin receptor and GPCR signaling systems by blocking YAP/TEAD-regulated gene expression in pancreatic cancer cells. Mol Cancer Res; 15(7); 929-41. (c)2017 AACR.
Biphasic Regulation of Yes-associated Protein (YAP) Cellular Localization, Phosphorylation, and Activity by G Protein-coupled Receptor Agonists in Intestinal Epithelial Cells: A NOVEL ROLE FOR PROTEIN KINASE D (PKD).[Pubmed:27369082]
J Biol Chem. 2016 Aug 19;291(34):17988-8005.
We examined the regulation of Yes-associated protein (YAP) localization, phosphorylation, and transcriptional activity in intestinal epithelial cells. Our results show that stimulation of intestinal epithelial IEC-18 cells with the G protein-coupled receptor (GPCR) agonist angiotensin II, a potent mitogen for these cells, induced rapid translocation of YAP from the nucleus to the cytoplasm (within 15 min) and a concomitant increase in YAP phosphorylation at Ser(127) and Ser(397) Angiotensin II elicited YAP phosphorylation and cytoplasmic accumulation in a dose-dependent manner (ED50 = 0.3 nm). Similar YAP responses were provoked by stimulation with vasopressin or serum. Treatment of the cells with the protein kinase D (PKD) family inhibitors CRT0066101 and kb NB 142-70 prevented the increase in YAP phosphorylation on Ser(127) and Ser(397) via Lats2, YAP cytoplasmic accumulation, and increase in the mRNA levels of YAP/TEAD-regulated genes (Ctgf and Areg). Furthermore, siRNA-mediated knockdown of PKD1, PKD2, and PKD3 markedly attenuated YAP nuclear-cytoplasmic shuttling, phosphorylation at Ser(127), and induction of Ctgf and Areg expression in response to GPCR activation. These results identify a novel role for the PKD family in the control of biphasic localization, phosphorylation, and transcriptional activity of YAP in intestinal epithelial cells. In turn, YAP and TAZ are necessary for the stimulation of the proliferative response of intestinal epithelial cells to GPCR agonists that act via PKD. The discovery of interaction between YAP and PKD pathways identifies a novel cross-talk in signal transduction and demonstrates, for the first time, that the PKDs feed into the YAP pathway.
Protein kinase D1 mediates class IIa histone deacetylase phosphorylation and nuclear extrusion in intestinal epithelial cells: role in mitogenic signaling.[Pubmed:24647541]
Am J Physiol Cell Physiol. 2014 May 15;306(10):C961-71.
We examined whether class IIa histone deacetylases (HDACs) play a role in mitogenic signaling mediated by protein kinase D1 (PKD1) in IEC-18 intestinal epithelial cells. Our results show that class IIa HDAC4, HDAC5, and HDAC7 are prominently expressed in these cells. Stimulation with ANG II, a potent mitogen for IEC-18 cells, induced a striking increase in phosphorylation of HDAC4 at Ser(246) and Ser(632), HDAC5 at Ser(259) and Ser(498), and HDAC7 at Ser(155). Treatment with the PKD family inhibitors kb NB 142-70 and CRT0066101 or small interfering RNA-mediated knockdown of PKD1 prevented ANG II-induced phosphorylation of HDAC4, HDAC5, and HDAC7. A variety of PKD1 activators in IEC-18 cells, including vasopressin, lysophosphatidic acid, and phorbol esters, also induced HDAC4, HDAC5, and HDAC7 phosphorylation. Using endogenously and ectopically expressed HDAC5, we show that PKD1-mediated phosphorylation of HDAC5 induces its nuclear extrusion into the cytoplasm. In contrast, HDAC5 with Ser(259) and Ser(498) mutated to Ala was localized to the nucleus in unstimulated and stimulated cells. Treatment of IEC-18 cells with specific inhibitors of class IIa HDACs, including MC1568 and TMP269, prevented cell cycle progression, DNA synthesis, and proliferation induced in response to G protein-coupled receptor/PKD1 activation. The PKD1-class IIa HDAC axis also functions in intestinal epithelial cells in vivo, since an increase in phosphorylation of HDAC4/5 and HDAC7 was demonstrated in lysates of crypt cells from PKD1 transgenic mice compared with matched nontransgenic littermates. Collectively, our results reveal a PKD1-class IIa HDAC axis in intestinal epithelial cells leading to mitogenic signaling.
Positive cross talk between protein kinase D and beta-catenin in intestinal epithelial cells: impact on beta-catenin nuclear localization and phosphorylation at Ser552.[Pubmed:26739494]
Am J Physiol Cell Physiol. 2016 Apr 1;310(7):C542-57.
Given the fundamental role of beta-catenin signaling in intestinal epithelial cell proliferation and the growth-promoting function of protein kinase D1 (PKD1) in these cells, we hypothesized that PKDs mediate cross talk with beta-catenin signaling. The results presented here provide several lines of evidence supporting this hypothesis. We found that stimulation of intestinal epithelial IEC-18 cells with the G protein-coupled receptor (GPCR) agonist angiotensin II (ANG II), a potent inducer of PKD activation, promoted endogenous beta-catenin nuclear localization in a time-dependent manner. A significant increase was evident within 1 h of ANG II stimulation (P< 0.01), peaked at 4 h (P< 0.001), and declined afterwards. GPCR stimulation also induced a marked increase in beta-catenin-regulated genes and phosphorylation at Ser(552) in intestinal epithelial cells. Exposure to preferential inhibitors of the PKD family (CRT006610 or kb NB 142-70) or knockdown of the isoforms of the PKD family prevented the increase in beta-catenin nuclear localization and phosphorylation at Ser(552) in response to ANG II. GPCR stimulation also induced the formation of a complex between PKD1 and beta-catenin, as shown by coimmunoprecipitation that depended on PKD1 catalytic activation, as it was abrogated by cell treatment with PKD family inhibitors. Using transgenic mice that express elevated PKD1 protein in the intestinal epithelium, we detected a marked increase in the localization of beta-catenin in the nucleus of crypt epithelial cells in the ileum of PKD1 transgenic mice, compared with nontransgenic littermates. Collectively, our results identify a novel cross talk between PKD and beta-catenin in intestinal epithelial cells, both in vitro and in vivo.
PKD1 mediates negative feedback of PI3K/Akt activation in response to G protein-coupled receptors.[Pubmed:24039875]
PLoS One. 2013 Sep 9;8(9):e73149.
We examined whether protein kinase D1 (PKD1) mediates negative feeback of PI3K/Akt signaling in intestinal epithelial cells stimulated with G protein-coupled receptor (GPCR) agonists. Exposure of intestinal epithelial IEC-18 cells to increasing concentrations of the PKD family inhibitor kb NB 142-70, at concentrations that inhibited PKD1 activation, strikingly potentiated Akt phosphorylation at Thr(308) and Ser(473) in response to the mitogenic GPCR agonist angiotensin II (ANG II). Enhancement of Akt activation by kb NB 142-70 was also evident in cells with other GPCR agonists, including vasopressin and lysophosphatidic acid. Cell treatment with the structurally unrelated PKD family inhibitor CRT0066101 increased Akt phosphorylation as potently as kb NB 142-70 [corrected]. Knockdown of PKD1 with two different siRNAs strikingly enhanced Akt phosphorylation in response to ANG II stimulation in IEC-18 cells. To determine whether treatment with kb NB 142-70 enhances accumulation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) in the plasma membrane, we monitored the redistribution of Akt-pleckstrin homology domain-green fluorescent protein (Akt-PH-GFP) in single IEC-18 cells. Exposure to kb NB 142-70 strikingly increased membrane accumulation of Akt-PH-GFP in response to ANG II. The translocation of the PIP3 sensor to the plasma membrane and the phosphorylation of Akt was completed prevented by prior exposure to the class I p110alpha specific inhibitor A66. ANG II markedly increased the phosphorylation of p85alpha detected by a PKD motif-specific antibody and enhanced the association of p85alpha with PTEN. Transgenic mice overexpressing PKD1 showed a reduced phosphorylation of Akt at Ser(473) in intestinal epithelial cells compared to wild type littermates. Collectively these results indicate that PKD1 activation mediates feedback inhibition of PI3K/Akt signaling in intestinal epithelial cells in vitro and in vivo.
Synthesis and Structure-Activity Relationships of Benzothienothiazepinone Inhibitors of Protein Kinase D.[Pubmed:21617763]
ACS Med Chem Lett. 2011 Feb 14;2(2):154-159.
Protein kinase D (PKD) is a member of a novel family of serine/threonine kinases that regulate fundamental cellular processes. PKD is implicated in the pathogenesis of several diseases, including cancer. Progress in understanding the biological functions and therapeutic potential of PKD has been hampered by the lack of specific inhibitors. The benzoxoloazepinolone CID755673 was recently identified as the first potent and selective PKD inhibitor. The study of structure-activity relationships (SAR) of this lead structure led to further improvements in PKD1 potency. We describe herein the synthesis and biological evaluation of novel benzothienothiazepinone analogs. We achieved a ten-fold increase in the in vitro PKD1 inhibitory potency for the second generation lead kb-NB142-70 and accomplished a transition to an almost equally potent novel pyrimidine scaffold, while maintaining excellent target selectivity. These promising results will guide the design of pharmacological tools to dissect PKD function and pave the way for the development of potential anti-cancer agents.
Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility.[Pubmed:20444281]
BMC Chem Biol. 2010 May 5;10:5.
BACKGROUND: Protein kinase D (PKD) has been implicated in a wide range of cellular processes and pathological conditions including cancer. However, targeting PKD therapeutically and dissecting PKD-mediated cellular responses remains difficult due to lack of a potent and selective inhibitor. Previously, we identified a novel pan-PKD inhibitor, CID755673, with potency in the upper nanomolar range and high selectivity for PKD. In an effort to further enhance its selectivity and potency for potential in vivo application, small molecule analogs of CID755673 were generated by modifying both the core structure and side-chains. RESULTS: After initial activity screening, five analogs with equal or greater potencies as CID755673 were chosen for further analysis: kb-NB142-70, kb-NB165-09, kb-NB165-31, kb-NB165-92, and kb-NB184-02. Our data showed that modifications to the aromatic core structure in particular significantly increased potency while retaining high specificity for PKD. When tested in prostate cancer cells, all compounds inhibited PMA-induced autophosphorylation of PKD1, with kb-NB142-70 being most active. Importantly, these analogs caused a dramatic arrest in cell proliferation accompanying elevated cytotoxicity when applied to prostate cancer cells. Cell migration and invasion were also inhibited by these analogs with varying potencies that correlated to their cellular activity. CONCLUSIONS: Throughout the battery of experiments, the compounds kb-NB142-70 and kb-NB165-09 emerged as the most potent and specific analogs in vitro and in cells. These compounds are undergoing further testing for their effectiveness as pharmacological tools for dissecting PKD function and as potential anti-cancer agents in the treatment of prostate cancer.


