RivastigmineCholinesterase inhibitor CAS# 123441-03-2 |
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Rocuronium
Catalog No.:BCC1906
CAS No.:143558-00-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123441-03-2 | SDF | Download SDF |
| PubChem ID | 77991 | Appearance | Powder |
| Formula | C14H22N2O2 | M.Wt | 250.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | S-Rivastigmine | ||
| Solubility | DMSO : ≥ 50 mg/mL (199.73 mM) *"≥" means soluble, but saturation unknown. | ||
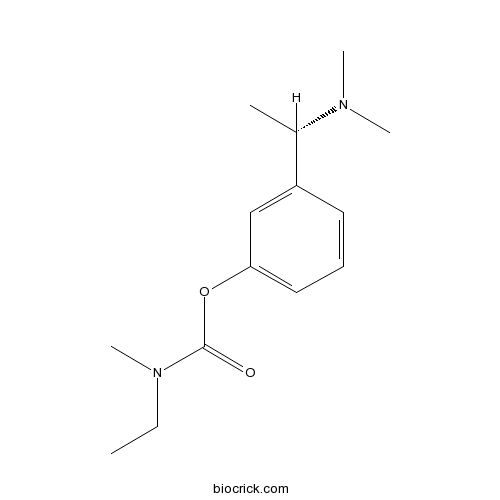
| Chemical Name | [3-[(1S)-1-(dimethylamino)ethyl]phenyl] N-ethyl-N-methylcarbamate | ||
| SMILES | CCN(C)C(=O)OC1=CC=CC(=C1)C(C)N(C)C | ||
| Standard InChIKey | XSVMFMHYUFZWBK-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rivastigmine, an cholinesterase inhibitor(IC50= 5.5 uM), inhibits both butyrylcholinesterase and acetylcholinesterase
IC50 value: 5.5 uM
Target: AChE
Rivastigmine is a parasympathomimetic or cholinergic agent for the treatment of mild to moderate dementia of the Alzheimer's type and dementia due to Parkinson's disease. The drug can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects, which typically include nausea and vomiting. The drug is eliminated through the urine, and appears to have relatively few drug-drug interactions. Rivastigmine, a cholinesterase inhibitor, inhibits both butyrylcholinesterase and acetylcholinesterase. It is thought to work by inhibiting these cholinesterase enzymes, which would otherwise break down the brain chemical acetylcholine. References: | |||||

Rivastigmine Dilution Calculator

Rivastigmine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9946 mL | 19.9728 mL | 39.9457 mL | 79.8913 mL | 99.8642 mL |
| 5 mM | 0.7989 mL | 3.9946 mL | 7.9891 mL | 15.9783 mL | 19.9728 mL |
| 10 mM | 0.3995 mL | 1.9973 mL | 3.9946 mL | 7.9891 mL | 9.9864 mL |
| 50 mM | 0.0799 mL | 0.3995 mL | 0.7989 mL | 1.5978 mL | 1.9973 mL |
| 100 mM | 0.0399 mL | 0.1997 mL | 0.3995 mL | 0.7989 mL | 0.9986 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rivastigmine tartrate, an cholinesterase inhibitor(IC50= 5.5 uM), inhibits both butyrylcholinesterase and acetylcholinesterase.
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Linderaspirone A
Catalog No.:BCN6122
CAS No.:1235126-46-1
- Azelnidipine
Catalog No.:BCC4400
CAS No.:123524-52-7
- AQ-RA 741
Catalog No.:BCC7314
CAS No.:123548-16-3
Enhancement of oral bioavailability of rivastigmine with quercetin nanoparticles by inhibiting CYP3A4 and esterases.[Pubmed:28189992]
Pharmacol Rep. 2017 Apr;69(2):365-370.
BACKGROUND: Quercetin is a well-known flavonoid, has pharmacokinetic interaction with ester drugs due to its capability of esterase inhibition in the gut and liver. However, the interaction between quercetin nanoparticles (NQC) and Rivastigmine has not been reported. Hence, the present study was performed to evaluate the effect of quercetin alone and its nanoparticles on the pharmacokinetics of Rivastigmine in rats. METHODS: NQC prepared by antisolvent precipitation method. The influence of quercetin on the pharmacokinetics of Rivastigmine was evaluated by following methods i.e. in vitro inhibitory effect on esterase enzyme in rat liver microsomes and in vitro assessment of CYP3A activity using erythromycin-N-demethylase (EMD) assay. To confirm these findings, an in vivo pharmacokinetic study of orally administered Rivastigmine in rats with quercetin and NQC pretreatments was performed. RESULTS: The size of NQC was observed below 300nm. Quercetin significantly (p<0.05) inhibited the esterase-mediated metabolism of Rivastigmine. In in vitro assessment of CYP3A activity model the erythromycin-N-demethylation (EMD) levels in quercetin treated group were significantly reduced (p<0.05). Cmax, AUC0-t and AUC0- infinity of Rivastigmine were found to be increased in quercetin and NQC pretreated groups. Further, the CL/F and Vd/F of Rivastigmine were significantly decreased. CONCLUSIONS: The results revealed that enhanced bioavailability of Rivastigmine might be caused by the combination of their effects due to CYP3A and esterase inhibition, Therefore, concomitant administration of NQC influences the bioavailability of Rivastigmine and also has synergetic effect in the treatment of Alzheimer's disease.
Development of a Discriminative In Vitro Release Test for Rivastigmine Transdermal Patches Using Pharmacopeial Apparatuses: USP 5 and USP 6.[Pubmed:28224389]
AAPS PharmSciTech. 2017 Oct;18(7):2561-2569.
The aim of this study was to develop and validate a discriminating in vitro release test to evaluate Rivastigmine transdermal patches. The Exelon(R) Patch was chosen as a model transdermal product. The studies of in vitro release were designed to determine the impact of the official apparatus chosen (USP apparatus 5 and USP apparatus 6), the rotation speed, and the dissolution medium characteristics on the Rivastigmine release profile from transdermal patches. Patches with different drug release profiles were tested in order to evaluate the discriminating power of the in vitro release test developed and validated. Variables such as the apparatus type, the dissolution medium, and the rotation speed have a significant influence on the drug release characteristics from a transdermal patch. The in vitro release methodologies using the USP apparatus 5 at 50 rpm and USP apparatus 6 at 25 rpm using the medium phosphate-buffered saline pH 7.4 were considered discriminative and adequate to characterize the Rivastigmine (RV) release from a commercial transdermal patch, Exelon(R) Patch.
The First Use of Pralidoxime in a Child With Rivastigmine Poisoning.[Pubmed:28328690]
Pediatr Emerg Care. 2018 Oct;34(10):e184-e186.
The aim of this report is to describe the successful use of pralidoxime in a pediatric patient who accidentally ingested 12 mg of Rivastigmine and presented to the emergency department with weakness, drowsiness, hyporeactivity to environmental stimuli, and full cholinergic syndrome. CASE: The patient presented to the emergency department 2 hours after a suspected ingestion of Rivastigmine. He was sleepy but oriented and cooperative, hypotonic, and hyporeflexic and has a Glasgow Coma Scale score of 13 (E3M6V4). Laboratory tests showed a low plasma cholinesterase levels of 2141 U/L (reference range, 5300-12 900 U/L), hyperglycemia (251 mg/dL), and leukocytosis with neutrophilia (21 900/mL, 75.2% neutrophils). CONCLUSIONS: Only 2 pediatric cases of Rivastigmine poisoning have been reported in the literature, and there are no previous reports of using pralidoxime in the management of this poisoning. In the present case, intravenous pralidoxime (30 mg/kg) was administered twice at the fifth and sixth hours of ingestion for nicotinic and central effects. There is reasonable theoretical science to suggest pralidoxime in case of acetylcholinesterase inhibitor toxicity. We conclude that observed clinical improvement in weakness temporally associated with pralidoxime administration. Increased plasma cholinesterase activity after pralidoxime administration also makes it useful in this type of poisoning.


