LRRK2-IN-1LRRK2 inhibitor,cell-permeable and ATP competitive CAS# 1234480-84-2 |
- HG-10-102-01
Catalog No.:BCC4271
CAS No.:1351758-81-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1234480-84-2 | SDF | Download SDF |
| PubChem ID | 46843906 | Appearance | Powder |
| Formula | C31H38N8O3 | M.Wt | 570.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (87.61 mM) *"≥" means soluble, but saturation unknown. | ||
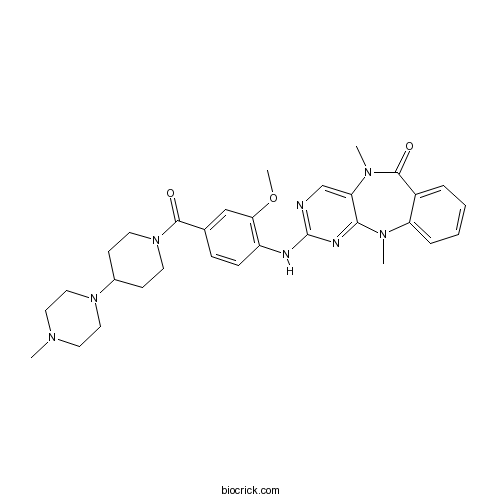
| Chemical Name | 2-[2-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidine-1-carbonyl]anilino]-5,11-dimethylpyrimido[4,5-b][1,4]benzodiazepin-6-one | ||
| SMILES | CN1CCN(CC1)C2CCN(CC2)C(=O)C3=CC(=C(C=C3)NC4=NC=C5C(=N4)N(C6=CC=CC=C6C(=O)N5C)C)OC | ||
| Standard InChIKey | IWMCPJZTADUIFX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H38N8O3/c1-35-15-17-38(18-16-35)22-11-13-39(14-12-22)29(40)21-9-10-24(27(19-21)42-4)33-31-32-20-26-28(34-31)36(2)25-8-6-5-7-23(25)30(41)37(26)3/h5-10,19-20,22H,11-18H2,1-4H3,(H,32,33,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of leucine-rich repeat kinase 2 (LRRK2). Inhibits both G2019S mutant and wild-type LRRK2 kinase activity (IC50 values are 6 and 13 nM respectively). Causes dephosphorylation, ubiquitination and degradation of LRRK2. |

LRRK2-IN-1 Dilution Calculator

LRRK2-IN-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7522 mL | 8.7612 mL | 17.5223 mL | 35.0447 mL | 43.8059 mL |
| 5 mM | 0.3504 mL | 1.7522 mL | 3.5045 mL | 7.0089 mL | 8.7612 mL |
| 10 mM | 0.1752 mL | 0.8761 mL | 1.7522 mL | 3.5045 mL | 4.3806 mL |
| 50 mM | 0.035 mL | 0.1752 mL | 0.3504 mL | 0.7009 mL | 0.8761 mL |
| 100 mM | 0.0175 mL | 0.0876 mL | 0.1752 mL | 0.3504 mL | 0.4381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LRRK2-IN-1 is a potent and selective inhibitor of LRRK2 with IC50 value of 13 nM. [1]
LRRK2 (Leucine-rich repeat kinase 2) is also known dardarin. LRRK2 belongs to the leucine-rich repeat kinase family. LRRK2 mostly located in the cytoplasm, however, it also associates with outer membrane of the mitochondrial. LRRK2 interacts with parkin at the C-terminal R2 RING finger domain. Parkin interacted with LRRK2 at the COR domain. LRRK2 mutation will induce apoptotic cell death of neuroblastoma cells. Expression of LRRK2 mutants has a close relationship with autosomal dominant Parkinson's disease. The LRRK2 Gly2019Ser mutation is a common cause of familial Parkinson's disease. The Gly2019Ser mutation has been proved to cause Parkinson's disease, even though it is a small number of LRRK2 mutations. LRRK2 also has relationship with Crohn's disease by genomewide association studies.
LRRK2-IN-1 dose-dependent inhibit the activity of wild-type and G2019S LRRK2 with IC50 of 0.17 and 0.04μM respectively in HEK293 cells stably expressing GFP tagged wild-type or mutated LRRK2.[2] LRRK2-IN-1 inhibits the activity of both wild-type and G2019S mutant LRRK2 with IC 50 values of 13 nM and 6 nM respectively in vitro enzyme assay with 0.1 mM ATP. LRRK2-IN-1 competed with ATP. LRRK2-IN-1 has a selective profile compared with other 442 diverse kinases and it has no inhibition effect with LRRK1. LRRK2-IN-1 induced endogenous LRRK2 phosphorylation in lymphoblastoid cells. LRRK2-IN-1 also induced Ser910/Ser935 dephosphorylation of LRRK2 in mice kidney at 100 mg/kg.[1]
References:
[1]. Deng X, Dzamko N, Prescott A, Davies P, Liu Q, Yang Q, Lee JD, Patricelli MP, Nomanbhoy TK, Alessi DR et al: Characterization of a selective inhibitor of the Parkinson's disease kinase LRRK2. Nat Chem Biol, 7(4):203-205.
[2]. Hermanson SB, Carlson CB, Riddle SM, Zhao J, Vogel KW, Nichols RJ, Bi K: Screening for novel LRRK2 inhibitors using a high-throughput TR-FRET cellular assay for LRRK2 Ser935 phosphorylation. PLoS One, 7(8):e43580.
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Linderaspirone A
Catalog No.:BCN6122
CAS No.:1235126-46-1
- Azelnidipine
Catalog No.:BCC4400
CAS No.:123524-52-7
- AQ-RA 741
Catalog No.:BCC7314
CAS No.:123548-16-3
- rac BHFF
Catalog No.:BCC7644
CAS No.:123557-91-5
- Peptide YY(3-36), PYY, human
Catalog No.:BCC1041
CAS No.:123583-37-9
Alternative to LRRK2-IN-1 for Pharmacological Studies of Parkinson's Disease.[Pubmed:26382237]
Pharmacology. 2015;96(5-6):240-7.
BACKGROUND/AIMS: LRRK2 (leucine-rich repeat protein kinase 2) is one of the most commonly accepted genes associated with Parkinson's disease (PD). The overexpression of disease-associated mutations in LRRK2 is toxic to the cells, while reduction or elimination of LRRK2 expression promotes cell health and growth. Thus, the identification of an LRRK2 inhibitor with good physiochemical and pharmacokinetic properties is of great interest for the treatment of PD. METHODS: In this study, we have investigated LRRK2 compounds, LRRK2-IN-1 and Compound 1, in vitro and in vivo to determine how suitable they are as a selective LRRK2 tool compound. RESULTS: We report that Compound 1, patented by GSK, is a potent and selective LRRK2 inhibitor with good blood-brain barrier permeability as reflected by its high brain to plasma ratio in rats. In addition, Compound 1 can significantly promote neurite outgrowth in a primary cortical culture, indicating an optimistic cellular function of this compound in a biological system. In contrast, LRRK2-IN-1 is a less selective LRRK2 inhibitor and has low brain penetration. Furthermore, LRRK2-IN-1 is cyto- and genotoxic, while Compound 1 does not exhibit any toxicity. CONCLUSIONS: These results suggest that Compound 1 may be a superior tool compound than LRRK2-IN-1 to advance future pharmacological research on LRRK2.
Synthesis and In Vitro and In Vivo Evaluation of [(3)H]LRRK2-IN-1 as a Novel Radioligand for LRRK2.[Pubmed:28289968]
Mol Imaging Biol. 2017 Dec;19(6):837-845.
PURPOSE: LRRK2 (leucine-rich repeat kinase 2) has recently been proven to be a promising drug target for Parkinson's disease (PD) due to an apparent enhanced activity caused by mutations associated with familial PD. To date, there have been no reports in which a LRRK2 inhibitor has been radiolabeled and used for in in vitro or in vivo studies of LRRK2. In the present study, we radiolabeled the LRRK2 ligand, LRRK-IN-1, for the purposes of performing in vitro (IC50, K d , B max, autoradiography) and in vivo (biodistribution, and blocking experiments) evaluations in rodents and human striatum tissues. PROCEDURES: [(3)H]LRRK2-IN-1 was prepared with high radiochemical purity (>99 %) and a specific activity of 41 Ci/mmol via tritium/hydrogen (T/H) exchange using Crabtree's catalyst. For IC(50), K d , and B max determination, LRRK2-IN-1 was used as a competing drug for nonspecific binding assessment. The specific binding of the tracer was further evaluated via an in vivo blocking study in mice with a potent LRRK2 inhibitor, Pf-06447475. RESULTS: In vitro binding studies demonstrated a saturable binding site for [(3)H]LRRK2-IN-1 in rat kidney, rat brain striatum and human brain striatum with K d of 26 +/- 3 and 43 +/- 8, 48 +/- 2 nM, respectively. In rat, the density of LRRK2 binding sites (B max) was higher in kidney (6.4 +/- 0.04 pmol/mg) than in brain (2.5 +/- 0.03 pmol/mg), however, in human brain striatum, the B max was 0.73 +/- 0.01 pmol/mg protein. Autoradiography imaging in striatum of rat and human brain tissues gave results consistent with binding studies. In in vivo biodistribution and blocking studies in mice, co-administration with Pf-06447475 (10 mg/kg) reduced the uptake of [(3)H]LRRK2-IN-1 (%ID/g) by 50-60% in the kidney or brain. CONCLUSION: The high LRRK2 brain density observed in our study suggests the feasibility for positron emission tomography imaging of LRRK2 (a potential target) with radioligands of higher affinity and specificity.
Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent activity against colorectal and pancreatic cancer through inhibition of doublecortin-like kinase 1.[Pubmed:24885928]
Mol Cancer. 2014 May 6;13:103.
BACKGROUND: Doublecortin-like kinase 1 (DCLK1) is emerging as a tumor specific stem cell marker in colorectal and pancreatic cancer. Previous in vitro and in vivo studies have demonstrated the therapeutic effects of inhibiting DCLK1 with small interfering RNA (siRNA) as well as genetically targeting the DCLK1+ cell for deletion. However, the effects of inhibiting DCLK1 kinase activity have not been studied directly. Therefore, we assessed the effects of inhibiting DCLK1 kinase activity using the novel small molecule kinase inhibitor, LRRK2-IN-1, which demonstrates significant affinity for DCLK1. RESULTS: Here we report that LRRK2-IN-1 demonstrates potent anti-cancer activity including inhibition of cancer cell proliferation, migration, and invasion as well as induction of apoptosis and cell cycle arrest. Additionally we found that it regulates stemness, epithelial-mesenchymal transition, and oncogenic targets on the molecular level. Moreover, we show that LRRK2-IN-1 suppresses DCLK1 kinase activity and downstream DCLK1 effector c-MYC, and demonstrate that DCLK1 kinase activity is a significant factor in resistance to LRRK2-IN-1. CONCLUSIONS: Given DCLK1's tumor stem cell marker status, a strong understanding of its biological role and interactions in gastrointestinal tumors may lead to discoveries that improve patient outcomes. The results of this study suggest that small molecule inhibitors of DCLK1 kinase should be further investigated as they may hold promise as anti-tumor stem cell drugs.
LRRK2 dephosphorylation increases its ubiquitination.[Pubmed:25939886]
Biochem J. 2015 Jul 1;469(1):107-20.
Activating mutations in the leucine rich repeat protein kinase 2 (LRRK2) gene are the most common cause of inherited Parkinson's disease (PD). LRRK2 is phosphorylated on a cluster of phosphosites including Ser(910), Ser(935), Ser(955) and Ser(973), which are dephosphorylated in several PD-related LRRK2 mutants (N1437H, R1441C/G, Y1699C and I2020T) linking the regulation of these sites to PD. These serine residues are also dephosphorylated after kinase inhibition and lose 14-3-3 binding, which serves as a pharmacodynamic marker for inhibited LRRK2. Loss of 14-3-3 binding is well established, but the consequences of dephosphorylation are only now being uncovered. In the present study, we found that potent and selective inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(935) then ubiquitination and degradation of a significant fraction of LRRK2. GNE1023 treatment decreased the phosphorylation and stability of LRRK2 in expression systems and endogenous LRRK2 in A549 cells and in mouse dosing studies. We next established that LRRK2 is ubiquitinated through at least Lys(48) and Lys(63) ubiquitin linkages in response to inhibition. To investigate the link between dephosphorylation induced by inhibitor treatment and LRRK2 ubiquitination, we studied LRRK2 in conditions where it is dephosphorylated such as expression of PD mutants [R1441G, Y1699C and I2020T] or by blocking 14-3-3 binding to LRRK2 via difopein expression, and found LRRK2 is hyper-ubiquitinated. Calyculin A treatment prevents inhibitor and PD mutant induced dephosphorylation and reverts LRRK2 to a lesser ubiquitinated species, thus directly implicating phosphatase activity in LRRK2 ubiquitination. This dynamic dephosphorylation-ubiquitination cycle could explain detrimental loss-of-function phenotypes found in peripheral tissues of LRRK2 kinase inactive mutants, LRRK2 KO (knockout) animals and following LRRK2 inhibitor administration.
Characterization of a selective inhibitor of the Parkinson's disease kinase LRRK2.[Pubmed:21378983]
Nat Chem Biol. 2011 Apr;7(4):203-5.
Mutations in leucine-rich repeat kinase 2 (LRRK2) are strongly associated with late-onset autosomal dominant Parkinson's disease. We employed a new, parallel, compound-centric approach to identify a potent and selective LRRK2 inhibitor, LRRK2-IN-1, and demonstrated that inhibition of LRRK2 induces dephosphorylation of Ser910 and Ser935 and accumulation of LRRK2 within aggregate structures. LRRK2-IN-1 will serve as a versatile tool to pharmacologically interrogate LRRK2 biology and study its role in Parkinson's disease.


