Biperiden HClAnticholinergic drug CAS# 1235-82-1 |
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- Biotin-HPDP
Catalog No.:BCC3583
CAS No.:129179-83-5
- NHS-SS-Biotin
Catalog No.:BCC3581
CAS No.:142439-92-7
- Sulfo-NHS-SS-Biotin
Catalog No.:BCC3580
CAS No.:325143-98-4
- NHS-LC-Biotin
Catalog No.:BCC3579
CAS No.:72040-63-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1235-82-1 | SDF | Download SDF |
| PubChem ID | 92151 | Appearance | Powder |
| Formula | C21H30ClNO | M.Wt | 347.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Akineton | ||
| Solubility | H2O : 5 mg/mL (14.37 mM; Need ultrasonic) | ||
| Chemical Name | α-Bicyclo[2.2.1]hept-5-en-2-yl-α-phen | ||
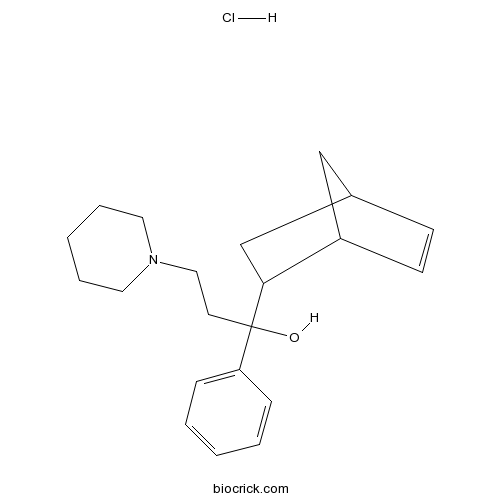
| SMILES | [H+].[Cl-].OC(CCN1CCCCC1)(C2CC3CC2C=C3)c4ccccc4 | ||
| Standard InChIKey | RDNLAULGBSQZMP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H29NO.ClH/c23-21(19-7-3-1-4-8-19,11-14-22-12-5-2-6-13-22)20-16-17-9-10-18(20)15-17;/h1,3-4,7-10,17-18,20,23H,2,5-6,11-16H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Muscarinic receptor antagonist that displays some selectivity for the M1 subtype (Ki values are 0.48, 2.4, 3.9, 6.3 and 6.3 nM for M1, M4, M3, M2 and M5 receptors respectively). Alleviates extrapyramidal symptoms associated with antipsychotic drugs. Displays antiparkinsonian activity. |

Biperiden HCl Dilution Calculator

Biperiden HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8742 mL | 14.3711 mL | 28.7422 mL | 57.4845 mL | 71.8556 mL |
| 5 mM | 0.5748 mL | 2.8742 mL | 5.7484 mL | 11.4969 mL | 14.3711 mL |
| 10 mM | 0.2874 mL | 1.4371 mL | 2.8742 mL | 5.7484 mL | 7.1856 mL |
| 50 mM | 0.0575 mL | 0.2874 mL | 0.5748 mL | 1.1497 mL | 1.4371 mL |
| 100 mM | 0.0287 mL | 0.1437 mL | 0.2874 mL | 0.5748 mL | 0.7186 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Biperiden HCl is an anticholinergic drug [1].
As an anticholinergic drug, biperiden is first used in the treatment of Parkinson's disease. It is a competitive antagonist of muscarinic receptor. It has been believed that in the patients of PD, a reduction of intranigral dopamine concentrations results in a relative imbalance between the dopaminergic and cholinergic neurological pathways. The anticholinergics can correct the imbalance through reducing the degree of neurotransmission mediated by neostriatal acetylcholine. Biperiden is also used to treat extrapyramidal side effects of antipsychotic drugs. In addition, the misuse of biperiden causing delirium has been reported in several clinical settings [1, 2].
References:
[1] Brocks D R. Anticholinergic drugs used in Parkinson’s disease: an overlooked class of drugs from a pharmacokinetic perspective. J Pharm Pharm Sci, 1999, 2(2): 39-46.
[2] Espi Martinez F, Espi Forcen F, Shapov A, et al. Biperiden Dependence: Case Report and Literature Review. Case reports in psychiatry, 2012, 2012.
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- Linderaspirone A
Catalog No.:BCN6122
CAS No.:1235126-46-1
- Azelnidipine
Catalog No.:BCC4400
CAS No.:123524-52-7
- AQ-RA 741
Catalog No.:BCC7314
CAS No.:123548-16-3
- rac BHFF
Catalog No.:BCC7644
CAS No.:123557-91-5
- Peptide YY(3-36), PYY, human
Catalog No.:BCC1041
CAS No.:123583-37-9
- Curcumadionol
Catalog No.:BCN3561
CAS No.:1235984-45-8
- PHP 501 trifluoroacetate
Catalog No.:BCC6193
CAS No.:1236105-75-1
- 740 Y-P
Catalog No.:BCC5861
CAS No.:1236188-16-1
- Clerosterol glucoside
Catalog No.:BCN6123
CAS No.:123621-00-1
- Salviaplebeiaside
Catalog No.:BCN7304
CAS No.:1236273-88-3
- Diosbulbin L
Catalog No.:BCN7305
CAS No.:1236285-87-2
- Tenofovir maleate
Catalog No.:BCC4262
CAS No.:1236287-04-9
Efficacy of biperiden and atropine as anticonvulsant treatment for organophosphorus nerve agent intoxication.[Pubmed:10877003]
Arch Toxicol. 2000 May;74(3):165-72.
The ability of the nerve agents tabun, sarin, soman, GF, VR, and VX to produce brain seizures and the effectiveness of the anticholinergics Biperiden HCl or atropine SO4 as an anticonvulsant treatment were studied in a guinea-pig model. All animals were implanted a week prior to the experiment with cortical electrodes for electroencephalogram (EEG) recordings. On the day of exposure, the animals were pretreated with pyridostigmine (0.026 mg/kg, i.m.) 30 min prior to challenge with a 2 x LD50 dose (s.c.) of a given agent. In separate experiments, animals were challenged with 5 x LD50 (s.c.) of soman. One minute after agent challenge, the animals were treated intramuscularly (i.m.) with 2 mg/kg atropine SO4 admixed with 25 mg/kg 2-PAM Cl and then observed for the onset of seizure activity. Five minutes after the start of nerve agent-induced EEG seizures, animals were treated i.m. with different doses of Biperiden HCl or atropine SO4 and observed for seizure termination. The anticonvulsant ED50 of Biperiden HCl and atropine SO4 for termination of seizures induced by each nerve agent was calculated and compared. With equally toxic doses (2 x LD50) of these agents, continuous EEG seizures (status epilepticus) developed in all animals challenged with soman, tabun, or VR, and in more than 90% of the animals challenged with GF or sarin. In contrast, only 50% of the animals developed seizures when challenged with VX. The times to onset of seizures for soman, tabun, GF, and sarin were very similar (5-8 min) while for VR, it was about 10 min. In the case of VX, not only was the time to seizure development longer (20.7 min), but the seizure activity in 19% of the animals terminated spontaneously within 5 min after onset and did not return. Under these conditions, the anticonvulsant ED50s of Biperiden HCl for soman, GF, VR, tabun, sarin, and VX were 0.57, 0.51, 0.41, 0.2, 0.1, and 0.09 mg/kg, respectively, while those of atropine SO4 for soman, VR, tabun, GF, sarin, and VX were 12.2, 11.9, 10.4, 10.3, 5.1, and 4.1 mg/kg, respectively. In separate experiments, the anticonvulsant ED50 doses of biperiden for animals challenged with 2 or 5 x LD50 of soman were 0.48 (95% confidence limits 0.25-0.73) or 0.57 (95% CI 0.38-0.84) mg/kg, respectively, while the anticonvulsant ED50s for atropine (12.2 mg/kg, i.m.) were identical under these same two challenge conditions. The present study demonstrates that all nerve agents can produce status epilepticus and that the therapeutic effectiveness of atropine and biperiden roughly paralleled the seizurogenic potential of these agents.
Mood elevating effect of trihexyphenidyl and biperiden in individuals taking antipsychotic medication.[Pubmed:852367]
Dis Nerv Syst. 1977 May;38(5):353-5.
The author calls attention to mood-elevation as a side effect of Biperiden HCl and Trihexyphenidyl HCL, two anticholinergic antiparkinsonian agents. This is of significance because of the despondency and anergy often seen in schizophrenics taking antipsychotic medication and because of the difficulty discerning the origin of affective changes in the face of polypharmacy.
Muscarinic receptor occupancy by biperiden in living human brain.[Pubmed:10069534]
Life Sci. 1999;64(8):PL99-104.
Anticholinergic drug is often used to treat extrapyramidal symptoms. We measured muscarinic cholinergic receptor (mAchR) occupancy by the oral administration of biperiden in eight healthy subjects using positron emission tomography (PET) and [11C]N-methyl-4-piperidylbenzilate (NMPB). After the baseline scan each subject underwent one or two post-dose PET scans. mAchR occupancy was 10-45% in the frontal cortex three hours after the oral administration of 4 mg of biperiden. The occupancy correlated with the plasma concentration of biperiden in a curvilinear manner.
Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells.[Pubmed:1346637]
J Pharmacol Exp Ther. 1992 Feb;260(2):576-80.
We determined the affinity and selectivity of binding for 24 compounds: nine antimuscarinics (including some antiparkinson drugs) and 15 neuroleptics (including the atypical compounds clozapine, fluperlapine, melperone, rilapine, risperidone, tenilapine, tiosperone and zotepine) at the five human muscarinic receptor subtypes expressed in Chinese hamster ovary cells. Equilibrium dissociation constants (Kd) were obtained from competitive radioligand binding studies with [3H]quinuclidinyl benzilate and membranal preparations of these cells. As expected, QNB had the highest affinity of the compounds studied at the five receptor subtypes and was not selective (Kd ranged from 0.027-0.088 nM). Benztropine had the next highest affinity of the antimuscarinic compounds and thioridazine had the highest affinity of the neuroleptics. Among the antiparkinson drugs, biperiden was the only one selective for the m1 subtype; and among the neuroleptics, the atypical drug clozapine was also selective for the m1 subtype. This selectivity may explain clozapine's unusual efficacy in refractory schizophrenic patients and its low incidence of extrapyramidal side effects. However, because most other atypical neuroleptics studied lacked high affinity and selectivity at muscarinic receptor subtypes, it is likely that other mechanisms are involved as well.


