AzelnidipineL-type calcium channel blocker;antihypertensive CAS# 123524-52-7 |
- AST-1306 TsOH
Catalog No.:BCC4043
CAS No.:1050500-29-2
- Compound 56
Catalog No.:BCC3615
CAS No.:171745-13-4
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- AST-1306
Catalog No.:BCC3727
CAS No.:897383-62-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123524-52-7 | SDF | Download SDF |
| PubChem ID | 65948 | Appearance | Powder |
| Formula | C33H34N4O6 | M.Wt | 582.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (171.63 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
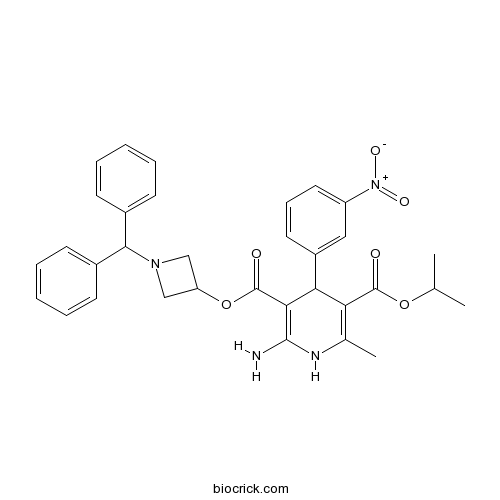
| Chemical Name | 3-O-(1-benzhydrylazetidin-3-yl) 5-O-propan-2-yl 2-amino-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | ||
| SMILES | CC1=C(C(C(=C(N1)N)C(=O)OC2CN(C2)C(C3=CC=CC=C3)C4=CC=CC=C4)C5=CC(=CC=C5)[N+](=O)[O-])C(=O)OC(C)C | ||
| Standard InChIKey | ZKFQEACEUNWPMT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C33H34N4O6/c1-20(2)42-32(38)27-21(3)35-31(34)29(28(27)24-15-10-16-25(17-24)37(40)41)33(39)43-26-18-36(19-26)30(22-11-6-4-7-12-22)23-13-8-5-9-14-23/h4-17,20,26,28,30,35H,18-19,34H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Azelnidipine Dilution Calculator

Azelnidipine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7163 mL | 8.5815 mL | 17.163 mL | 34.3259 mL | 42.9074 mL |
| 5 mM | 0.3433 mL | 1.7163 mL | 3.4326 mL | 6.8652 mL | 8.5815 mL |
| 10 mM | 0.1716 mL | 0.8581 mL | 1.7163 mL | 3.4326 mL | 4.2907 mL |
| 50 mM | 0.0343 mL | 0.1716 mL | 0.3433 mL | 0.6865 mL | 0.8581 mL |
| 100 mM | 0.0172 mL | 0.0858 mL | 0.1716 mL | 0.3433 mL | 0.4291 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Azelnidipine is a novel dihydropyridine derivative, a L-type calcium channel blocker, and an antihypertensive. Acute administration of azelnidipine prevents a sudden drop of cardiac function after acute stress.
- Linderaspirone A
Catalog No.:BCN6122
CAS No.:1235126-46-1
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- AQ-RA 741
Catalog No.:BCC7314
CAS No.:123548-16-3
- rac BHFF
Catalog No.:BCC7644
CAS No.:123557-91-5
- Peptide YY(3-36), PYY, human
Catalog No.:BCC1041
CAS No.:123583-37-9
- Curcumadionol
Catalog No.:BCN3561
CAS No.:1235984-45-8
- PHP 501 trifluoroacetate
Catalog No.:BCC6193
CAS No.:1236105-75-1
- 740 Y-P
Catalog No.:BCC5861
CAS No.:1236188-16-1
- Clerosterol glucoside
Catalog No.:BCN6123
CAS No.:123621-00-1
- Salviaplebeiaside
Catalog No.:BCN7304
CAS No.:1236273-88-3
- Diosbulbin L
Catalog No.:BCN7305
CAS No.:1236285-87-2
- Tenofovir maleate
Catalog No.:BCC4262
CAS No.:1236287-04-9
- Fmoc-Glu(OBzl)-OH
Catalog No.:BCC3493
CAS No.:123639-61-2
- NS 398
Catalog No.:BCC6857
CAS No.:123653-11-2
Solid state characterization of azelnidipine-oxalic acid co-crystal and co-amorphous complexes: The effect of different azelnidipine polymorphs.[Pubmed:28237872]
J Pharm Biomed Anal. 2017 May 10;138:302-315.
In present study, based on the two polymorphs (alpha and beta form) of Azelnidipine (AZE), 12 complexes of AZE and oxalic acid (OXA) were prepared by solvent-assisted grinding (SG) and neat powder grinding (NG) methods at the AZE/OXA molar ratios of 2:1, 1:1, and 1:2. The effect of the different polymorphs of AZE on the micro-structure of the complexes were investigated by powder X-ray diffraction (PXRD), tempreture modulated differential scanning calorimetry and thermogravimetric analysis, cryo-field emission scanning electron microscope system, fourier transform infrared (FTIR), and solid-state nuclear magnetic resonance spectroscopy. beta-AZE-OXA co-crystal was produced at beta-AZE/OXA molar ratio of 2:1 when SG method was used; while alpha-AZE was used to produce alpha-AZE-OXA co-crystal at same condition. However, the other 10 combinations were in co-amorphous forms, including the NG samples with alpha (or beta)-AZE/OXA molar ratios of 2:1, 1:1 (SG and NG), and 1:2 (SG and NG). Although the XRD pattern and IR spectra of the two co-crystals showed no difference, the melting enthalpy and specific heat cp of the beta-AZE-OXA co-crystal was higher than that of the alpha-AZE-OXA co-crystal, indicating that the numbers of solvent molecules which entered the two co-crystal lattices were different. Interestingly, obvious difference occurred in the IR spectra between the alpha-AZE-OXA and beta-AZE-OXA co-amorphous systems. 1745cm(-1) wave-numbers, which were assigned to the free CO groups, appeared in the alpha-AZE-OXA co-amorphous systems even when just a small amount of OXA was introduced, thereby indicating the presence of different intermolecular forces in the two series of co-amorphous forms. The solubility in different media and the dissolution rate in 0.1molL(-1) HCl of the 12 complexes were determined. The dramatically improved dissolution rates of the alpha- and beta-AZE-OXA 1:2 (NG) combinations in vitro showed potential in improving the physicochemical properties of AZE by co-amorphous complex formation.
Comparative effect of fixed-dose combination tablets of candesartan cilexetil/amlodipine versus olmesartan medoxomil/azelnidipine on laboratory parameters in patients with hypertension: a retrospective cohort study.[Pubmed:26453437]
Clin Exp Hypertens. 2016;38(2):173-9.
We conducted a retrospective cohort study to evaluate and compare the long-term effects of two single-pill fixed-dose combinations (FDCs), candesartan/amlodipine and olmesartan/Azelnidipine, on laboratory parameters in patients in routine clinical practice. We identified an equal number of new users (n = 182) of a candesartan/amlodipine (8/5 mg/day) FDC tablet (CAN/AML users) and a propensity-score matched cohort (n = 182) receiving an olmesartan/Azelnidipine (20/16 mg/day) FDC tablet (OLM/AZ users). Generalized estimating equations were used to estimate and compare the effects of the drugs on serum levels of creatinine, estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN), uric acid, sodium, potassium, aspartate aminotransferase, and alanine aminotransferase levels up to 12 months after the start of study drug administration. There was a significant increase of serum creatinine level and a significant decrease of eGFR from the baseline period to during the exposure period in both CAN/AML and OLM/AZ users, and a significant increase of BUN level in CAN/AML users. However, there were no significant differences in the mean changes of laboratory parameters between CAN/AML and OLM/AZ users. Our findings suggested that the effects of CAN/AML and OLM/AZ on laboratory parameters, including an unfavorable effect on renal function, were similar at least during 1 year of administration.
Olmesartan with azelnidipine versus with trichlormethiazide on home blood pressure variability in patients with type II diabetes mellitus.[Pubmed:28089902]
J Am Soc Hypertens. 2017 Mar;11(3):140-147.
The aim of the present study was to compare the effects of olmesartan combined with Azelnidipine versus olmesartan combined with trichlormethiazide, on home blood pressure (BP) and pressure variability in type II diabetes mellitus patients using home BP telemonitoring system. We performed an open-label cross-over pilot study of 28 patients with type II diabetes mellitus. Patients received combination treatment with either olmesartan 20 mg plus Azelnidipine 16 mg or olmesartan 20 mg plus trichlormethiazide 1 mg for more than 6 weeks each in a cross-over method. The coefficient of morning systolic BP variability in the olmesartan plus Azelnidipine group was significantly lower than that in the olmesartan plus trichlormethiazide group (6.4 +/- 1.9 vs. 7.5 +/- 2.6, P = .004). There were no significant differences in mean morning systolic BP between the two groups. Using home BP telemonitoring for hypertensive patients with type II diabetes, this study revealed for the first time that the olmesartan with Azelnidipine combination is superior to the olmesartan with trichlormethiazide combination in reducing home BP variability.
Effects of azelnidipine and amlodipine on exercise-induced sympathoexcitation assessed by pupillometry in hypertensive patients.[Pubmed:27439493]
Hypertens Res. 2016 Dec;39(12):863-867.
The pupil is a suitable end organ for studying autonomic function because both sympathetic and parasympathetic nerve activity can be evaluated independently using a light stimulus. Sympathetic response elicited by physical stress is augmented in hypertensive patients compared with normotensive subjects, which increases the risk of cardiovascular events. We used pupillometry to evaluate the effects of the calcium channel blockers Azelnidipine (AZ) and amlodipine (AM) on changes in autonomic nervous activity induced by isometric exercise in patients with hypertension. Twenty patients with essential hypertension who were administered AM and 21 who were administered AZ underwent a pupillary function test and blood pressure (BP) and pulse rate (PR) measurements before and after isometric handgrip exercise (IHG). Maximal velocities of pupil constriction (VC) and re-dilation (VD) obtained with light stimulation for 1 s were used as indices of parasympathetic and sympathetic nerve activity, respectively. Increases in systolic BP and PR elicited by IHG were significantly smaller in the AZ group than in the AM group. After IHG, both VC and VD significantly increased in the AM group but not in the AZ group. The low-to-high frequency ratio obtained from analysis of PR variability, another measure of sympathetic activity, also increased in only the AM group. Thus AZ inhibited autonomic activation and suppressed cardiovascular responses to IHG more effectively than AM. The sympathoinhibitory effect of AZ may therefore be beneficial for patients with essential hypertension. In addition, pupillometry was shown to be a useful tool for assessing autonomic function in hypertensive patients.


