Z-Arg-OHCAS# 1234-35-1 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

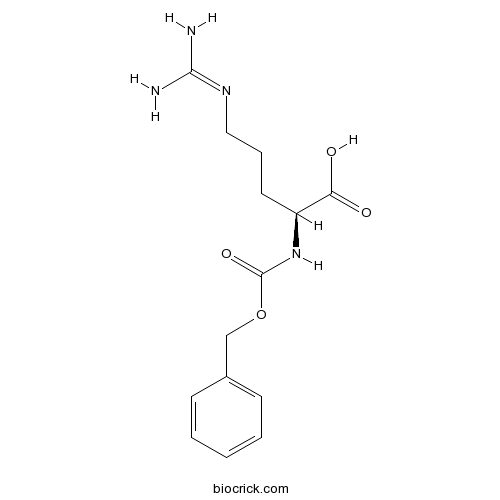
| Cas No. | 1234-35-1 | SDF | Download SDF |
| PubChem ID | 71055 | Appearance | Powder |
| Formula | C14H20N4O4 | M.Wt | 308.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 1234-35-1; Z-Arg-OH; Nalpha-Cbz-L-arginine; Nalpha-Carbobenzyloxy-L-arginine | ||
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | (2S)-5-(diaminomethylideneamino)-2-(phenylmethoxycarbonylamino)pentanoic acid | ||
| SMILES | C1=CC=C(C=C1)COC(=O)NC(CCCN=C(N)N)C(=O)O | ||
| Standard InChIKey | SJSSFUMSAFMFNM-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C14H20N4O4/c15-13(16)17-8-4-7-11(12(19)20)18-14(21)22-9-10-5-2-1-3-6-10/h1-3,5-6,11H,4,7-9H2,(H,18,21)(H,19,20)(H4,15,16,17)/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Arg-OH Dilution Calculator

Z-Arg-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2436 mL | 16.218 mL | 32.4359 mL | 64.8719 mL | 81.0898 mL |
| 5 mM | 0.6487 mL | 3.2436 mL | 6.4872 mL | 12.9744 mL | 16.218 mL |
| 10 mM | 0.3244 mL | 1.6218 mL | 3.2436 mL | 6.4872 mL | 8.109 mL |
| 50 mM | 0.0649 mL | 0.3244 mL | 0.6487 mL | 1.2974 mL | 1.6218 mL |
| 100 mM | 0.0324 mL | 0.1622 mL | 0.3244 mL | 0.6487 mL | 0.8109 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Arg-OH
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- LY2835219 free base
Catalog No.:BCC1722
CAS No.:1231929-97-7
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
Conformational study of Z-Glu-OH and Z-Arg-OH: dispersion interactions versus conventional hydrogen bonding.[Pubmed:23095122]
J Phys Chem A. 2013 Feb 14;117(6):1216-27.
The gas-phase conformational preferences of the model dipeptides Z-Glu-OH and Z-Arg-OH have been studied in the low-temperature environment of a supersonic jet. IR-UV ion-dip spectra obtained using the free electron laser FELIX provide conformation-specific IR spectra, which in combination with density functional theory (DFT) allow us to determine the conformational structures of the peptides. Molecular dynamics modeling using simulated annealing generates a variety of low-energy structures, for which geometry optimization and frequency calculations are then performed using the B3LYP functional with the 6-311+G(d,p) basis set. By comparing experimental and theoretical IR spectra, three conformations for Z-Glu-OH and two for Z-Arg-OH have been identified. For three of the five structures, the dispersion interaction provides an important contribution to the stabilization, emphasizing the importance of these forces in small peptides. Therefore, dispersion-corrected DFT functionals (M05-2X and B97D) have also been employed in our theoretical analysis. Second-order Moller-Plesset perturbation theory (MP2) has been used as benchmark for the relative energies of the different conformational structures. Finally, we address the ongoing debate on the gas-phase structure of arginine by elucidating whether isolated arginine is canonical, tautomeric, or zwitterionic.


