BazedoxifeneEstrogen receptor modulator CAS# 198481-32-2 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 198481-32-2 | SDF | Download SDF |
| PubChem ID | 154257 | Appearance | Powder |
| Formula | C30H34N2O3 | M.Wt | 470.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
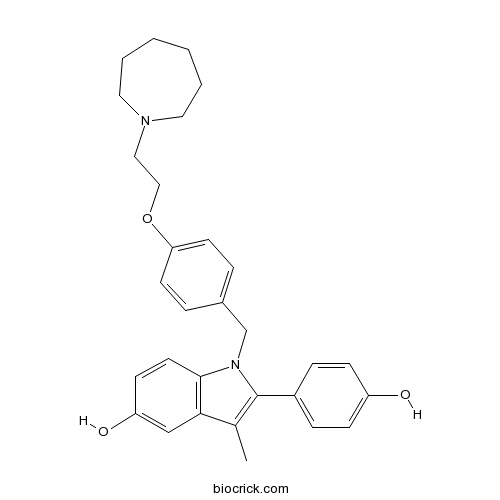
| Chemical Name | 1-[[4-[2-(azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methylindol-5-ol | ||
| SMILES | CC1=C(N(C2=C1C=C(C=C2)O)CC3=CC=C(C=C3)OCCN4CCCCCC4)C5=CC=C(C=C5)O | ||
| Standard InChIKey | UCJGJABZCDBEDK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H34N2O3/c1-22-28-20-26(34)12-15-29(28)32(30(22)24-8-10-25(33)11-9-24)21-23-6-13-27(14-7-23)35-19-18-31-16-4-2-3-5-17-31/h6-15,20,33-34H,2-5,16-19,21H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bazedoxifene is a third generation selective modulator of estrogen receptor. | |||||
| Targets | estrogen receptor | |||||

Bazedoxifene Dilution Calculator

Bazedoxifene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1249 mL | 10.6247 mL | 21.2495 mL | 42.4989 mL | 53.1237 mL |
| 5 mM | 0.425 mL | 2.1249 mL | 4.2499 mL | 8.4998 mL | 10.6247 mL |
| 10 mM | 0.2125 mL | 1.0625 mL | 2.1249 mL | 4.2499 mL | 5.3124 mL |
| 50 mM | 0.0425 mL | 0.2125 mL | 0.425 mL | 0.85 mL | 1.0625 mL |
| 100 mM | 0.0212 mL | 0.1062 mL | 0.2125 mL | 0.425 mL | 0.5312 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bazedoxifene is a selective estrogen receptor modulator (SERM) with IC50 value of 26 and 99 nM for estrogen receptorα and β, respectively [1].
Bazedoxifene is a SERM used for the prevention and treatment of post menopausalo steoporosis which is caused by the loss of endogenous levels of estrogens. It is developed as a third-generation SERM which can reduce the vertebral and nonvertebral fracture risk and have no stimulatory effects on breast or uterus. Bazedoxifene is an estrogen receptor ligand with an indole-based core binding domain. It binds to both ERα (IC50 value of 23nM) and ERβ (IC50 value of 85nM), exhibited agonist activity in bone, cardiovascular system and CNS while exhibited antagonist activity in breast and endometrium. It competitively inhibited the binding of 17β-estradiol to both ERα and ERβ [1, 2 and 3].
In MCF7 cells, treatment of bazedoxifene showed no ER agonist activity and no effect on the cell proliferation. However, it suppressed the 17β-estradiol -induced transcriptional activation and cell proliferation [3].
In ovariectomized rats, administration of bazedoxifene at dose of 0.3 or 3.0 mg /kg daily for 6 weeks resulted in the protection of skeleton from bone loss with increased bone mineraldensity. In addition, the compression strength of the fifth lumbar vertebra was significantly improved by bazedoxifene. Moreover, administration of bazedoxifene at dose of3.0 mg /kg/day showed modest stimulatory effects on rat uterine with small increase in wet uterine weight. Bazedoxifene also had no ER agonist effects on rat vasomotor activity [2 and 3].
References:
[1] de Villiers TJ. Bazedoxifene: a novel selective estrogen receptor modulator for postmenopausal osteoporosis. Climacteric. 2010 Jun;13(3):210-8.
[2] Duggan ST, McKeage K. Bazedoxifene: a review of its use in the treatment of postmenopausal osteoporosis. Drugs. 2011 Nov 12;71(16):2193-212.
[3] Kawate H, Takayanagi R. Efficacy and safety of bazedoxifene for postmenopausal osteoporosis. Clin Interv Aging. 2011;6:151-60.
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Boc-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC2623
CAS No.:198474-61-2
- Parecoxib Sodium
Catalog No.:BCC4248
CAS No.:198470-85-8
- Parecoxib
Catalog No.:BCC4041
CAS No.:198470-84-7
- Boc-D-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC3308
CAS No.:198470-36-9
- LY 367385
Catalog No.:BCC6983
CAS No.:198419-91-9
- Erythrodiol 3-palmitate
Catalog No.:BCN4869
CAS No.:19833-13-7
- Myricetin 3-O-beta-D-glucopyranoside
Catalog No.:BCN8144
CAS No.:19833-12-6
- Medicagol
Catalog No.:BCN8430
CAS No.:1983-72-8
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
- GW311616
Catalog No.:BCC5393
CAS No.:198062-54-3
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- 9,10-Anthracenedione
Catalog No.:BCN3469
CAS No.:19852-76-7
- Fmoc-Asparaginol(Trt)
Catalog No.:BCC3042
CAS No.:198543-08-7
- Fmoc-HoTyr-OH.DCHA
Catalog No.:BCC3246
CAS No.:198560-10-0
- Fmoc-Ser(tBu)-ol
Catalog No.:BCC2578
CAS No.:198561-87-4
- Tranylcypromine hydrochloride
Catalog No.:BCC7791
CAS No.:1986-47-6
- Alisol A 23-acetate
Catalog No.:BCN3457
CAS No.:19865-75-9
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
- Cabralealactone
Catalog No.:BCN4870
CAS No.:19865-87-3
- MLCK inhibitor peptide
Catalog No.:BCC5852
CAS No.:198694-74-5
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
- Bavachinin
Catalog No.:BCN4871
CAS No.:19879-30-2
Bazedoxifene Ameliorates Homocysteine-Induced Apoptosis and Accumulation of Advanced Glycation End Products by Reducing Oxidative Stress in MC3T3-E1 Cells.[Pubmed:27832315]
Calcif Tissue Int. 2017 Mar;100(3):286-297.
Elevated plasma homocysteine (Hcy) level increases the risk of osteoporotic fracture by deteriorating bone quality. However, little is known about the effects of Hcy on osteoblast and collagen cross-links. This study aimed to investigate whether Hcy induces apoptosis of osteoblastic MC3T3-E1 cells as well as affects enzymatic and nonenzymatic collagen cross-links and to determine the effects of Bazedoxifene, a selective estrogen receptor modulator, on the Hcy-induced apoptosis and deterioration of collagen cross-links in the cells. Hcy treatments (300 muM, 3 mM, and 10 mM) increased intracellular reactive oxygen species (ROS) production in a dose-dependent manner. Propidium iodide staining showed that 3 and 10 mM Hcy induced apoptosis of MC3T3-E1 cells. Moreover, the activities of caspases-8, 9, and 3 were increased by 3 mM Hcy. The detrimental effects of 3 mM Hcy on apoptosis and ROS production were partly reversed by Bazedoxifene and 17beta estradiol. In addition, real-time PCR, immunostaining and Western blot showed that 300 muM Hcy decreased the expression of lysyl oxidase (Lox). Furthermore, 300 muM Hcy increased extracellular accumulation of pentosidine, an advanced glycation end product. Treatment with Bazedoxifene ameliorated Hcy-induced suppression of Lox expression and increase in pentosidine accumulation. These findings suggest that high-dose Hcy induces apoptosis of osteoblasts by increasing oxidative stress, and low-dose Hcy decreases enzymatic collagen cross-links and increases pentosidine accumulation, resulting in the deterioration of bone quality. Bazedoxifene treatment effectively prevents the Hcy-induced detrimental reactions of osteoblasts. Thus, Bazedoxifene may be a potent therapeutic drug for preventing Hcy-induced bone fragility.
The evolving role of oral hormonal therapies and review of conjugated estrogens/bazedoxifene for the management of menopausal symptoms.[Pubmed:28132583]
Postgrad Med. 2017 Apr;129(3):340-351.
This review describes the evolving role of oral hormone therapy (HT) for treating menopausal symptoms and preventing osteoporosis, focusing on conjugated estrogens/Bazedoxifene (CE/BZA). Estrogens alleviate hot flushes and prevent bone loss associated with menopause. In nonhysterectomized women, a progestin should be added to estrogens to reduce the risk of endometrial cancer. Use of HT declined since the Women's Health Initiative (WHI) studies showed that HT does not prevent coronary heart disease (CHD) and that conjugated estrogens/medroxyprogesterone acetate increased the risk of invasive breast cancer after nearly 5 years of use. However, re-analyses of the WHI data suggest that some risks (eg, CHD, all-cause mortality) may be reduced when HT is initiated in women <60 years of age and <10 years since menopause, compared with later. CE/BZA is the first menopausal HT without a progestogen for nonhysterectomized women. Instead, BZA, a selective estrogen receptor modulator, in combination with CE, protects against estrogenic effects on uterine and breast tissue. Data from 5 large, randomized clinical trials show that CE/BZA reduces hot flush frequency/severity, prevents bone loss, reduces bone turnover, improves the vaginal maturation index and ease of lubrication, and improves some measures of sleep and menopause-specific quality of life. In studies of up to 2 years, there was no increase in endometrial hyperplasia, vaginal bleeding, breast density, or breast pain/tenderness compared with placebo. Venous thromboembolism and stroke are risks of all estrogen-based therapies. The choice of HT should be individualized, with consideration of the risk/benefit profile and tolerability of therapy, as well as patient preferences.
Direct and indirect effects of conjugated estrogens/bazedoxifene treatment on quality of life in postmenopausal women.[Pubmed:27823739]
Maturitas. 2016 Dec;94:173-179.
OBJECTIVE: Determine the direct and indirect effects of conjugated estrogens/Bazedoxifene (CE/BZA) treatment on menopause-specific quality of life in a post-hoc analysis of 2 phase 3 Selective estrogens, Menopause, And Response to Therapy (SMART-1, SMART-2) trials. STUDY DESIGN: Data from participants in SMART-1 and SMART-2 who received CE 0.45mg/BZA 20mg, CE 0.625mg/BZA 20mg, or placebo were analyzed, including week 12 data from 1274 healthy postmenopausal women in SMART-1 and pooled week 1-4 and 9-12 data from 332 women with moderate to severe vasomotor symptoms (>/=7 HFs/d or >/=50/wk) in SMART-2. MAIN OUTCOME MEASURES: A statistical mediation model included treatment as a binary predictor variable (pooled active treatment vs placebo), Menopause-Specific Quality of Life (MENQOL) questionnaire domains (vasomotor, sexual, psychosocial, and physical function) as the outcomes variables, and frequency/severity of hot flushes (HFs) as treatment effect mediators on MENQOL. RESULTS: Vasomotor function was affected directly (SMART-1: 35.8%, P<0.05; SMART-2: 48.7%, P<0.05)] by CE/BZA and indirectly through improvements in HF severity (57.0%, P<0.05; 42.1%, P<0.05), and to a lesser extent, through reduction in HF frequency (7.2%, P<0.05; 9.2%, P<0.05). The effects of CE/BZA on the sexual, psychosocial, and physical function domains were fully mediated via improvements in HF severity. Final models for both studies were similar, indicating that direct and indirect effects of CE/BZA on menopause-specific quality of life are consistent and stable in different samples of women. CONCLUSIONS: CE/BZA affected the MENQOL vasomotor domain both directly and indirectly, whereas effects on other domains were fully mediated via HF severity reductions.
Bazedoxifene as a Novel GP130 Inhibitor for Pancreatic Cancer Therapy.[Pubmed:27535971]
Mol Cancer Ther. 2016 Nov;15(11):2609-2619.
The IL6/GP130/STAT3 pathway is crucial for tumorigenesis in multiple cancer types, including pancreatic cancer, and presents as a viable target for cancer therapy. We reported Bazedoxifene, which is approved as a selective estrogen modulator by FDA, as a novel inhibitor of IL6/GP130 protein-protein interactions using multiple ligand simultaneous docking and drug repositioning approaches. STAT3 is one of the major downstream effectors of IL6/GP130. Here, we observed Bazedoxifene inhibited STAT3 phosphorylation and STAT3 DNA binding, induced apoptosis, and suppressed tumor growth in pancreatic cancer cells with persistent IL6/GP130/STAT3 signaling in vitro and in vivo In addition, IL6, but not INFgamma, rescued Bazedoxifene-mediated reduction of cell viability. Bazedoxifene also inhibited STAT3 phosphorylation induced by IL6 and IL11, but not by OSM or STAT1 phosphorylation induced by INFgamma in pancreatic cancer cells, suggesting that Bazedoxifene inhibits the GP130/STAT3 pathway mediated by IL6 and IL11. Furthermore, Bazedoxifene combined with paclitaxel or gemcitabine synergistically inhibited cell viability and cell migration in pancreatic cancer cells. These results indicate that Bazedoxifene is a potential agent and can generate synergism when combined with conventional chemotherapy in human pancreatic cancer cells and tumor xenograft in mice. Therefore, our results support that Bazedoxifene as a novel inhibitor of GP130 signaling and may be a potential and safe therapeutic agent for human pancreatic cancer therapy. Mol Cancer Ther; 15(11); 2609-19. (c)2016 AACR.


