GW311616CAS# 198062-54-3 |
- ARP 100
Catalog No.:BCC2370
CAS No.:704888-90-4
- Alvelestat
Catalog No.:BCC4058
CAS No.:848141-11-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 198062-54-3 | SDF | Download SDF |
| PubChem ID | 9800961 | Appearance | Powder |
| Formula | C19H31N3O4S | M.Wt | 397.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 44 mg/mL (110.68 mM) *"≥" means soluble, but saturation unknown. | ||
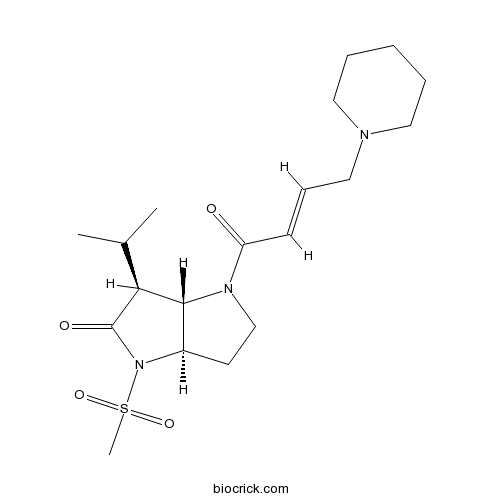
| Chemical Name | (3aR,6S,6aS)-4-methylsulfonyl-1-[(E)-4-piperidin-1-ylbut-2-enoyl]-6-propan-2-yl-3,3a,6,6a-tetrahydro-2H-pyrrolo[3,2-b]pyrrol-5-one | ||
| SMILES | CC(C)C1C2C(CCN2C(=O)C=CCN3CCCCC3)N(C1=O)S(=O)(=O)C | ||
| Standard InChIKey | NDNKNUMSTIMSHQ-URZKGLGPSA-N | ||
| Standard InChI | InChI=1S/C19H31N3O4S/c1-14(2)17-18-15(22(19(17)24)27(3,25)26)9-13-21(18)16(23)8-7-12-20-10-5-4-6-11-20/h7-8,14-15,17-18H,4-6,9-13H2,1-3H3/b8-7+/t15-,17+,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of human leukocyte elastase (HLE) (IC50 = 22 nM); displays > 4500-fold selectivity over other human serine proteases. Orally active in vivo. |

GW311616 Dilution Calculator

GW311616 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5155 mL | 12.5777 mL | 25.1553 mL | 50.3107 mL | 62.8883 mL |
| 5 mM | 0.5031 mL | 2.5155 mL | 5.0311 mL | 10.0621 mL | 12.5777 mL |
| 10 mM | 0.2516 mL | 1.2578 mL | 2.5155 mL | 5.0311 mL | 6.2888 mL |
| 50 mM | 0.0503 mL | 0.2516 mL | 0.5031 mL | 1.0062 mL | 1.2578 mL |
| 100 mM | 0.0252 mL | 0.1258 mL | 0.2516 mL | 0.5031 mL | 0.6289 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GW311616 is a potent, intracellular, orally bioavailable, long duration inhibitor of human neutrophil elastase(HNE) with IC50 of 22 nM; free base form of GW311616A. IC50 value: 22 nM [1] Target: neutrophil elastase The HNE inhibitor GW311616A is selective over other human serine proteases (IC50 values >100 uM for trypsin, cathepsin G, and plasmin, >3 mM for chymotrypsin and tissue plasminogen activator). Acetylcholinesterase is not inhibited by GW311616A at 100 uM.GW311616A is more potent than thetrifluoromethylketone inhibitor ZD8321 (Ki=13 nM). GW311616A is orallybioavailable in rat, dog (Table 4) and hamster despite moderate to high plasma clearance, which indicates that clearance is predominantly extrahepatic.
References:
[1]. Macdonald SJ, et al. The discovery of a potent, intracellular, orally bioavailable, long duration inhibitor of human neutrophil elastase--GW311616A a development candidate. Bioorg Med Chem Lett. 2001 Apr 9;11(7):895-8.
- AM 404
Catalog No.:BCC6945
CAS No.:198022-70-7
- GW311616 hydrochloride
Catalog No.:BCC5394
CAS No.:197890-44-1
- Stachartin E
Catalog No.:BCN6970
CAS No.:1978388-58-7
- Stachartin D
Catalog No.:BCN6971
CAS No.:1978388-57-6
- Stachartin C
Catalog No.:BCN6972
CAS No.:1978388-56-5
- Stachartin B
Catalog No.:BCN6973
CAS No.:1978388-55-4
- Stachartin A
Catalog No.:BCN6974
CAS No.:1978388-54-3
- SN 003
Catalog No.:BCC7633
CAS No.:197801-88-0
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- 7-O-Acetylbonducellpin C
Catalog No.:BCN7558
CAS No.:197781-86-5
- Bonducellpin D
Catalog No.:BCN7544
CAS No.:197781-85-4
- Bonducellpin C
Catalog No.:BCN7647
CAS No.:197781-84-3
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- Medicagol
Catalog No.:BCN8430
CAS No.:1983-72-8
- Myricetin 3-O-beta-D-glucopyranoside
Catalog No.:BCN8144
CAS No.:19833-12-6
- Erythrodiol 3-palmitate
Catalog No.:BCN4869
CAS No.:19833-13-7
- LY 367385
Catalog No.:BCC6983
CAS No.:198419-91-9
- Boc-D-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC3308
CAS No.:198470-36-9
- Parecoxib
Catalog No.:BCC4041
CAS No.:198470-84-7
- Parecoxib Sodium
Catalog No.:BCC4248
CAS No.:198470-85-8
- Boc-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC2623
CAS No.:198474-61-2
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
Neutrophil elastase and its therapeutic effect on leukemia cells.[Pubmed:26081156]
Mol Med Rep. 2015 Sep;12(3):4165-4172.
Neutrophil elastase (NE) is an early myeloid-specific serine protease, which is predominantly produced by promyelocytes. A previous study demonstrated that NE has an important role in the development of acute promyelocytic leukemia (APL). The process of APL was shown to be accelerated in animals that expressed abundant NE, whereas NEdeficient mice were protected from APL development; thus suggesting an important role for NE in the development of APL. The present study aimed to investigate the effects and possible mechanisms of NE. Up- and downregulation of NE in various leukemia cell lines was conducted in order to explore its significance in the occurrence and procession of leukemia, with the aim of identifying novel targeted therapeutic drugs for the treatment of leukemia. NE was overexpressed in cells following infection with an adenovirus, and Cell Counting kit8 and flow cytometry results demonstrated that cell proliferation was promoted, and cell apoptosis was inhibited, as compared with the untreated cells. NE was downregulated in the cells by both RNA interference and treatment with GW311616A, a specific inhibitor of NE, following which cell growth was shown to be inhibited and apoptosis was induced. These results suggested that NE may promote the development of APL, therefore, NE may be a therapeutic target and its inhibitor GW311616A may be a potential therapeutic drug for leukemia. Furthermore, the apoptosisassociated protein Bcell lymphoma 2 (Bcl2)associated X protein was significantly increased, whereas Bcl2 was markedly decreased in the cells with downregulated NE. Further experiments revealed that the probable apoptosisassociated signaling pathway was the phosphoinositide 3kinase/AKT pathway. The present study is the first, to the best of our knowledge, to demonstrate that GW311616A, a specific NE inhibitor, may act as a potential targeted drug for leukemia, which may have a profound impact on the future of leukemia-targeted therapy.
[Neutrophil elastase inhibitor on proliferation and apoptosis of U937 cells].[Pubmed:23827109]
Zhonghua Xue Ye Xue Za Zhi. 2013 Jun;34(6):507-11.
OBJECTIVE: To study and compare the effect of neutrophil elastase inhibitors (GW311616A and sivelestat) on the proliferation and apoptosis of U937 cells. METHODS: Inhibitory effects of GW311616A and sivelestat on the proliferation of U937 cells were assayed by MTT assay. The morphologic changes of U937 cells were detected by transmission electron microscope, and apoptosis was observed by AnnexinV-FITC/PI staining. The changes of cell cycle and apoptosis were detected by flow cytometry. The expression of NE in U937 cells was observed by indirect immunofluorescence, the variations of content and activity of NE in U937 cells were measured through ELISA assay and colorimetric method. RESULTS: MTT showed that both NE inhibitors could inhibit the proliferation of U937 cells in a dose dependent manner. The IC50 of GW311616A and sivelestat were 150 and 214 mumol/L respectively. The inhibition effect of GW311616A was significantly higher than of sivelestat (P<0.01). Typical apoptosis morphological changes of U937 cells was observed through electron microscope. AnnexinV-FITC/PI staining showed that U937 cells could be induced to undergo apoptosis by the two inhibitors, the apoptosis ratio of 150mumol/L GW311616A group (13.60%) was significantly higher than that of 150mumol/L sivelestat group (3.69%)(P<0.01). The result of flow cytometry indicated that the apoptosis ratio of 150 mumol/L GW311616A group was 14.61%, U937 cell cycle was mainly blocked in G2/M phase; meanwhile 150 mumol/L sivelestat group as 4.25% with cell cycle in S phase. The fluorescence intensity of GW311616A group obviously decreased than of sivelestat group. And the two inhibitors could reduce the content and activity of NE in U937 cells, but the effect of GW311616A was significantly higher than of sivelestat (P<0.01). CONCLUSION: GW311616A and sivelestat could inhibit the proliferation and cause apoptosis of U937 cells. Furthermore, GW311616A was more effective and harmful to cells than sivelestat.
Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure.[Pubmed:23562077]
Cell Metab. 2013 Apr 2;17(4):534-48.
The molecular mechanisms involved in the development of obesity and related complications remain unclear. Here, we report that obese mice and human subjects have increased activity of neutrophil elastase (NE) and decreased serum levels of the NE inhibitor alpha1-antitrypsin (A1AT, SerpinA1). NE null (Ela2(-/-)) mice and A1AT transgenic mice were resistant to high-fat diet (HFD)-induced body weight gain, insulin resistance, inflammation, and fatty liver. NE inhibitor GW311616A reversed insulin resistance and body weight gain in HFD-fed mice. Ela2(-/-) mice also augmented circulating high molecular weight (HMW) adiponectin levels, phosphorylation of AMP-activated protein kinase (AMPK), and fatty acid oxidation (FAO) in the liver and brown adipose tissue (BAT) and uncoupling protein (UCP1) levels in the BAT. These data suggest that the A1AT-NE system regulates AMPK signaling, FAO, and energy expenditure. The imbalance between A1AT and NE contributes to the development of obesity and related inflammation, insulin resistance, and liver steatosis.
The discovery of a potent, intracellular, orally bioavailable, long duration inhibitor of human neutrophil elastase--GW311616A a development candidate.[Pubmed:11294386]
Bioorg Med Chem Lett. 2001 Apr 9;11(7):895-8.
The discovery of a potent intracellular inhibitor of human neutrophil elastase which is orally active and has a long duration of action is described. The pharmacodynamic and pharmacokinetic properties of a trans-lactam development candidate, GW311616A, are described.
The rapid identification of drug metabolites using capillary liquid chromatography coupled to an ion trap mass spectrometer.[Pubmed:10209877]
Rapid Commun Mass Spectrom. 1999;13(5):456-63.
Capillary liquid chromatography (LC) using a 320 microns column and a flow rate of 10 microL/min has been coupled to an ion trap mass spectrometer using electrospray ionisation (ESI) to enable the rapid and effective identification of metabolites in urine, following oral administration of a novel human neutrophil elastase inhibitor, GW311616. Metabolites were identified from their mass (MS) spectra and tandem (MS/MS) mass spectra using minimal sample (1 microL of urine) and no sample pretreatment. Sensitivity assessment has shown that both molecular weight and structural information is obtainable on as little as 5 pg of compound, making the capillary LC/ion trap system as described an ideal analytical tool for the detection and characterisation of low level metabolites in biofluids (particularly when sample volume is limited). This level of detection was unattainable using a triple quadrupole mass spectrometer operating in full-scan mode, although 200 fg on column was detected using selected reaction monitoring target analysis.


