TFLLR-NH2PAR1 selective agonist CAS# 197794-83-5 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
- AC 55541
Catalog No.:BCC3951
CAS No.:916170-19-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 197794-83-5 | SDF | Download SDF |
| PubChem ID | 10146183 | Appearance | Powder |
| Formula | C31H53N9O6 | M.Wt | 647.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
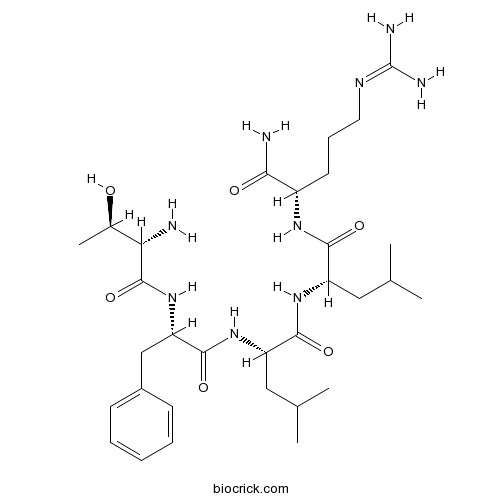
| Sequence | TFLLR (Modifications: Arg-5 = C-terminal amide) | ||
| Chemical Name | (2S)-N-[(2S)-1-[[(2S)-1-amino-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]-2-[[(2S)-2-[[(2S,3R)-2-amino-3-hydroxybutanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanamide | ||
| SMILES | CC(C)CC(C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)N)NC(=O)C(CC1=CC=CC=C1)NC(=O)C(C(C)O)N | ||
| Standard InChIKey | ANAMCEKSRDPIPX-GFGQVAFXSA-N | ||
| Standard InChI | InChI=1S/C31H53N9O6/c1-17(2)14-22(27(43)37-21(26(33)42)12-9-13-36-31(34)35)38-28(44)23(15-18(3)4)39-29(45)24(16-20-10-7-6-8-11-20)40-30(46)25(32)19(5)41/h6-8,10-11,17-19,21-25,41H,9,12-16,32H2,1-5H3,(H2,33,42)(H,37,43)(H,38,44)(H,39,45)(H,40,46)(H4,34,35,36)/t19-,21+,22+,23+,24+,25+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide derived from the protease-activated receptor-1 (PAR1) that acts as a PAR1 selective agonist (EC50 = 1.9 μM). Stimulates PAR1-mediated plasma extravasation in vivo. Control Peptide RLLFT-NH2 also available. |

TFLLR-NH2 Dilution Calculator

TFLLR-NH2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Peptide derived from the protease-activated receptor-1 (PAR1) that acts as a PAR1 selective agonist (EC50 = 1.9 μM). Stimulates PAR1-mediated plasma extravasation in vivo. Control Peptide RLLFT-NH2 also available.
- 7-O-Acetylbonducellpin C
Catalog No.:BCN7558
CAS No.:197781-86-5
- Bonducellpin D
Catalog No.:BCN7544
CAS No.:197781-85-4
- Bonducellpin C
Catalog No.:BCN7647
CAS No.:197781-84-3
- Daphmacrine
Catalog No.:BCN4868
CAS No.:19775-48-5
- Amiodarone HCl
Catalog No.:BCC4377
CAS No.:19774-82-4
- Peimisine
Catalog No.:BCN4992
CAS No.:19773-24-1
- Ajugatakasins A
Catalog No.:BCN3661
CAS No.:197723-20-9
- Loxapine
Catalog No.:BCC4026
CAS No.:1977-10-2
- Mesna
Catalog No.:BCC3811
CAS No.:19767-45-4
- Tigecycline hydrochloride
Catalog No.:BCC4228
CAS No.:197654-04-9
- Fmoc-N-Me-Ser(tBu)-OH
Catalog No.:BCC3353
CAS No.:197632-77-2
- Fmoc-D-β-HomoTrp(Boc)-OH
Catalog No.:BCC2624
CAS No.:197632-75-1
- SN 003
Catalog No.:BCC7633
CAS No.:197801-88-0
- Stachartin A
Catalog No.:BCN6974
CAS No.:1978388-54-3
- Stachartin B
Catalog No.:BCN6973
CAS No.:1978388-55-4
- Stachartin C
Catalog No.:BCN6972
CAS No.:1978388-56-5
- Stachartin D
Catalog No.:BCN6971
CAS No.:1978388-57-6
- Stachartin E
Catalog No.:BCN6970
CAS No.:1978388-58-7
- GW311616 hydrochloride
Catalog No.:BCC5394
CAS No.:197890-44-1
- AM 404
Catalog No.:BCC6945
CAS No.:198022-70-7
- GW311616
Catalog No.:BCC5393
CAS No.:198062-54-3
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- Medicagol
Catalog No.:BCN8430
CAS No.:1983-72-8
Microglia Activation and Polarization After Intracerebral Hemorrhage in Mice: the Role of Protease-Activated Receptor-1.[Pubmed:27206851]
Transl Stroke Res. 2016 Dec;7(6):478-487.
Polarized microglia play a dual (beneficial/detrimental) role in neurological diseases. However, the status and the factors that modulate microglia polarization in intracerebral hemorrhage (ICH) remain unclear. In the present study, we investigated the role of protease-activated receptor-1 (PAR-1, a thrombin receptor) in ICH-induced microglia polarization in mice. Male wild-type (WT) and PAR-1 knockout (PAR-1 KO) mice received an infusion of 30-muL autologous blood or saline into the right basal ganglia. Mice were euthanized at different time points and the brains were used for Western blotting and immunohistochemistry. Some mice had magnetic resonance imaging. We found that ICH induced microglia activation and polarization. M1 phenotypic markers were markedly increased and reached a peak as early as 4 h, remained high at 3 days and decreased 7 days after ICH. M2 phenotypic markers were upregulated later than M1 markers reaching a peak at day 1 and declining by day 7 after ICH. PAR-1 was upregulated after ICH and expressed in the neurons and microglia. ICH induced less brain swelling and neuronal death in PAR-1 KO mice, and this was associated with less M1 polarization and reduced proinflammatory cytokine levels in the brain. In conclusion, these results suggest that polarized microglia occur dynamically after ICH and that PAR-1 plays a role in the microglia activation and polarization.
Pharmacological Tools to Study the Role of Astrocytes in Neural Network Functions.[Pubmed:27714684]
Adv Exp Med Biol. 2016;949:47-66.
Despite that astrocytes and microglia do not communicate by electrical impulses, they can efficiently communicate among them, with each other and with neurons, to participate in complex neural functions requiring broad cell-communication and long-lasting regulation of brain function. Glial cells express many receptors in common with neurons; secrete gliotransmitters as well as neurotrophic and neuroinflammatory factors, which allow them to modulate synaptic transmission and neural excitability. All these properties allow glial cells to influence the activity of neuronal networks. Thus, the incorporation of glial cell function into the understanding of nervous system dynamics will provide a more accurate view of brain function. Our current knowledge of glial cell biology is providing us with experimental tools to explore their participation in neural network modulation. In this chapter, we review some of the classical, as well as some recent, pharmacological tools developed for the study of astrocyte's influence in neural function. We also provide some examples of the use of these pharmacological agents to understand the role of astrocytes in neural network function and dysfunction.
Dabigatran ameliorates post-haemorrhagic hydrocephalus development after germinal matrix haemorrhage in neonatal rat pups.[Pubmed:28155585]
J Cereb Blood Flow Metab. 2017 Sep;37(9):3135-3149.
We aim to determine if direct thrombin inhibition by dabigatran will improve long-term brain morphological and neurofunctional outcomes and if potential therapeutic effects are dependent upon reduced PAR-1 stimulation and consequent mTOR activation. Germinal matrix haemorrhage was induced by stereotaxically injecting 0.3 U type VII-S collagenase into the germinal matrix of P7 rat pups. Animals were divided into five groups: sham, vehicle (5% DMSO), dabigatran intraperitoneal, dabigatran intraperitoneal + TFLLR-NH2 (PAR-1 agonist) intranasal, SCH79797 (PAR-1 antagonist) intraperitoneal, and dabigatran intranasal. Neurofunctional outcomes were determined by Morris water maze, rotarod, and foot fault evaluations at three weeks. Brain morphological outcomes were determined by histological Nissl staining at four weeks. Expression levels of p-mTOR/p-p70s6k at three days and vitronectin/fibronectin at 28 days were quantified. Intranasal and intraperitoneal dabigatran promoted long-term neurofunctional recovery, improved brain morphological outcomes, and reduced intracranial pressure at four weeks after GMH. PAR-1 stimulation tended to reverse dabigatran's effects on post-haemorrhagic hydrocephalus development. Dabigatran also reduced expression of short-term p-mTOR and long-term extracellular matrix proteins, which tended to be reversed by PAR-1 agonist co-administration. PAR-1 inhibition alone, however, did not achieve the same therapeutic effects as dabigatran administration.
Regulation of the Pacemaker Activity of Colonic Interstitial Cells of Cajal by Protease-Activated Receptors: Involvement of Hyperpolarization-Activated Cyclic Nucleotide Channels.[Pubmed:27265408]
Pharmacology. 2016;98(3-4):171-82.
BACKGROUND AND PURPOSE: The exact mechanism of protease-activated receptors (PARs) on pacemaker activity of interstitial cells of Cajal (ICCs) has not been reported. We investigated the effects on pacemaker activity by the activation of PARs and its signal mechanisms in colonic ICCs. METHODS: The whole-cell patch-clamp technique, RT-PCR and Ca2+ imaging were used in cultured ICCs from mouse colon. RESULTS: PAR-1 and PAR-2 were expressed in Ano-1 positive ICCs. TFLLR-NH2 (a PAR-1 agonist) and trypsin (a PAR-2 agonist) depolarized the membrane and increased the pacemaker potential frequency. U-73122 (a phospholipase C (PLC) inhibitor) and thapsigargin (a Ca2+ ATPase inhibitor) suppressed the TFLLR-NH2- and trypsin-induced effects on pacemaker potential. TFLLR-NH2 and trypsin also increased intracellular Ca2+ ([Ca2+]i) intensity with increasing of Ca2+ oscillations. Genistein (a tyrosine kinase inhibitor), SP600125 (a JNK inhibitor), CsCl, ZD7288, clonidine (hyperpolarization-activated cyclic nucleotide (HCN) channel blockers), SQ-22536 and dideoxyadenosine (adenylate cyclase inhibitors) suppressed the increased pacemaker potential frequency without effects on depolarization of the membrane induced by TFLLR-NH2 and trypsin. CONCLUSION: These results suggest that activation of PAR-1 and PAR-2 modulates the pacemaker activity of colonic ICCs through the PLC-dependent [Ca2+]i release pathway. The increased pacemaker potential frequency by PAR-1 and PAR-2 was also dependent on tyrosine kinase, JNK, and HCN activation.
Suppression of ischaemia-induced injuries in rat brain by protease-activated receptor-1 (PAR-1) activating peptide.[Pubmed:27238976]
Eur J Pharmacol. 2016 Sep 5;786:36-46.
Ischaemic stroke has become one of the leading causes of death and disability worldwide. The role of protease activated receptor-1 (PAR-1) in this disease is uncertain. In the present study, the actions of a protease activated receptor-1 activating peptide (PAR-1 AP) SFLLRN-NH2 were investigated in an in vivo rat model of ischaemic stroke induced by middle cerebral artery occlusion (MCAO) and in an in vitro model induced by oxygen and glucose deprivation (OGD) in primary cultured rat embryonic cortical neurones. Rats subjected to MCAO exhibited increased brain infarct volume, oedema, and neurological deficit. Rat cortical neurones subjected to OGD showed increased lactate dehydrogenase, caspase-3 activity and TUNEL positive cells, whereas, mitochondrial membrane potential and cell viability were decreased. Furthermore, both models had elevated levels of reactive oxygen species, nitrite, and malondialdehyde, while anti-oxidant enzymes and bcl-2/bax ratio were decreased. These detrimental changes were suppressed by SFLLRN-NH2, and its protective actions were inhibited by a PAR-1 antagonist (BMS-200261). In summary, SFLLRN-NH2 was found to possess anti-oxidant and anti-apoptotic properties, and it produced marked inhibition on the detrimental effects of ischaemia in in vivo and in vitro models of ischaemic stroke. The present findings suggest PAR-1 is a promising target for development of novel treatments of ischaemic brain disease.
Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism.[Pubmed:11487506]
Br J Pharmacol. 2001 Aug;133(7):975-87.
Thrombin, generated in the circulation during injury, cleaves proteinase-activated receptor 1 (PAR1) to stimulate plasma extravasation and granulocyte infiltration. However, the mechanism of thrombin-induced inflammation in intact tissues is unknown. We hypothesized that thrombin cleaves PAR1 on sensory nerves to release substance P (SP), which interacts with the neurokinin 1 receptor (NK1R) on endothelial cells to cause plasma extravasation. PAR1 was detected in small diameter neurons known to contain SP in rat dorsal root ganglia by immunohistochemistry and in situ hybridization. Thrombin and the PAR1 agonist TFLLR-NH(2) (TF-NH(2)) increased [Ca(2+)](i) >50% of cultured neurons (EC(50)s 24 mu ml(-1) and 1.9 microM, respectively), assessed using Fura-2 AM. The PAR1 agonist completely desensitized responses to thrombin, indicating that thrombin stimulates neurons through PAR1. Injection of TF-NH(2) into the rat paw stimulated a marked and sustained oedema. An NK1R antagonist and ablation of sensory nerves with capsaicin inhibited oedema by 44% at 1 h and completely by 5 h. In wild-type but not PAR1(-/-) mice, TF-NH(2) stimulated Evans blue extravasation in the bladder, oesophagus, stomach, intestine and pancreas by 2 - 8 fold. Extravasation in the bladder, oesophagus and stomach was abolished by an NK1R antagonist. Thus, thrombin cleaves PAR1 on primary spinal afferent neurons to release SP, which activates the NK1R on endothelial cells to stimulate gap formation, extravasation of plasma proteins, and oedema. In intact tissues, neurogenic mechanisms are predominantly responsible for PAR1-induced oedema.
Characterization of the protease-activated receptor-1-mediated contraction and relaxation in the rat duodenal smooth muscle.[Pubmed:11065174]
Life Sci. 2000 Oct 6;67(20):2521-30.
Activation of protease-activated receptor-1 (PAR-1) produces a dual action, apamin-sensitive relaxation followed by contraction, in the rat duodenal smooth muscle, which is partially dependent on activation of L-type Ca2+ channels, protein kinase C (PKC) or tyrosine kinase (TK), and resistant to tetrodotoxin. The present study further characterized the PAR-1-mediated duodenal responses. Removal of extracellular Ca2+ as well as SK&F96365 reduced the contraction due to the PAR-1 agonist TFLLR-NH2 (TFp-NH2) by 60-80% that was similar to the extent of the inhibition by nifedipine. Lowering of the extracellular Na+ concentration, but not IAA-94, a Cl- channel inhibitor, reduced both the PAR-1-mediated contraction and relaxation by about 50%. U73122, a phospholipase C (PLC) inhibitor, or wortmannin, a phosphatidyl inositol 3'-kinase (PI3K) inhibitor, significantly reduced the PAR-1-mediated contraction, but not the relaxation, by itself, as the PKC inhibitor GF109203X and the TK inhibitor genistein did. U73122 or wortmannin, like GF109203X, when applied in combination with genistein, significantly reduced the PAR-1-mediated relaxation. The relaxation was resistant to antagonists of PACAP receptors, VIP receptors and P2 purinoceptors. Thus, the PAR-1-mediated contraction is considered to be dependent on intracellular and extracellular Ca2+, the influx of the latter being induced through activation of L-type Ca2+ channels triggered by the enhanced Na+ permeability, and that PLC and PI3K, in addition to PKC and TK, are involved in the PAR-1-mediated dual responses. Furthermore, non-adrenergic, non-cholinergic nerve neurotransmitter candidates that may modulate K+ channels do not appear to contribute to the relaxation by PAR-1 activation.
Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides.[Pubmed:9315351]
Can J Physiol Pharmacol. 1997 Jul;75(7):832-41.
We have used three distinct bioassay systems (rat aorta (RA) relaxation; rat gastric longitudinal muscle (LM) contraction; human embryonic kidney 293 (HEK293) cell calcium signal) to evaluate the activity and receptor selectivity of analogues of the receptor-activating peptides derived either from the thrombin receptor (TRAPs, based on the human receptor sequence, SFLLRNPNDK...) or the proteinase-activated receptor 2 (PAR2APs, based on the rat receptor sequence SLIGRL...). Our main focus was on the activation of PAR2 by PAR2APs and the cross-activation of PAR2 by the TRAPs. In the RA and LM assay systems, PAR2APs that were either N-acetylated (N-acetyl-SLIGRL-NH2) or had a reverse N-terminal sequence (LSIGRL-NH2) were inactive, either as agonists or antagonists. An alanine substitution at position 3 of the PAR2AP (SLAGRL-NH2) led to a dramatic reduction of biological activity, as did substitution of threonine for serine at position 1 (TLIGRL-NH2). However, alanine substitution at PAR2AP position 4 caused only a modest reduction in activity, resulting in a peptide (SLIARL-NH2) with a potency equivalent to that of the human PAR2AP, SLIGKV-NH2. The order of potency of the PAR2APs in the RA, LM, and HEK assay systems was SLIGRL-NH2 > SLIARL-NH2 > SLIGKV-NH2 > TLIGRL-NH2 > SLAGRL-NH2. In HEK cells, none of the PAR2APs activated the thrombin receptor (PAR1). However, in the HEK cell assay, the TRAP, SFLLR-NH2, activated or desensitized both PAR1 and PAR2 receptors, whereas the xenopus TRAP, TFRIFD-NH2, activated or desensitized selectively PAR1 but not PAR2. By constructing human-xenopus hybrid peptides, we found that the TRAPs, TFLLR-NH2, and SFLLFD-NH2 selectively activated the thrombin receptor in HEK cells without activating or desensitizing PAR2. In contrast, the TRAPs SFLLRD-NH2 and AFLLR-NH2 activated or desensitized both PAR1 and PAR2. The order of potency for the TRAPs in all bioassay systems was SFLLR-NH2 approximately equal to SFLLRD-NH2 approximately equal to TFLLR-NH2 > SFLLFD-NH2 > TFRIFD-NH2. We conclude that the N-terminal domain of the PAR2AP as well as positon 3 plays important roles for PAR2 activation. In contrast, the first and fifth amino acids in the TRAP motif, SFLLR-NH2, do not play a unique role in activating the thrombin receptor, but if appropriately modified can abrogate the ability of this peptide to cross-desensitize or activate PAR2, so as to be selective for PAR1. The PAR1- and PAR2-selective peptides that we have synthesized will be of use for the evaluation of the roles of the PAR1 and PAR2 receptor systems in vivo.


