ML161PAR1 inhibitor CAS# 423735-93-7 |
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

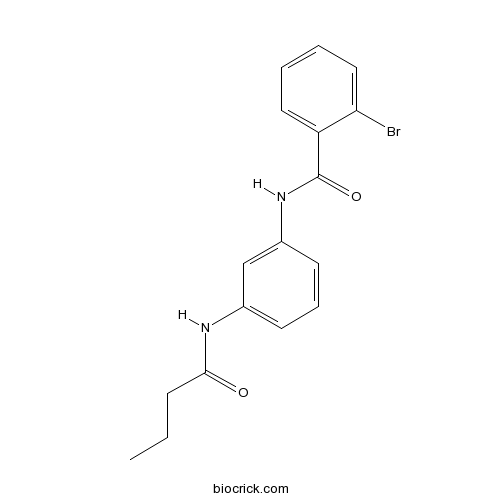
| Cas No. | 423735-93-7 | SDF | Download SDF |
| PubChem ID | 1048267 | Appearance | Powder |
| Formula | C17H17BrN2O2 | M.Wt | 361.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (276.83 mM; Need ultrasonic) | ||
| Chemical Name | 2-bromo-N-[3-(butanoylamino)phenyl]benzamide | ||
| SMILES | CCCC(=O)NC1=CC=CC(=C1)NC(=O)C2=CC=CC=C2Br | ||
| Standard InChIKey | DFOVLSMXPWPCFH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H17BrN2O2/c1-2-6-16(21)19-12-7-5-8-13(11-12)20-17(22)14-9-3-4-10-15(14)18/h3-5,7-11H,2,6H2,1H3,(H,19,21)(H,20,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of protease-activated receptor 1 (PAR1)-mediated platelet activation (IC50 = 0.26 μM for the inhibition of platelet P-selectin expression on human platelets). Thought to act allosterically. Also inhibits thrombin-induced platelet activation. |

ML161 Dilution Calculator

ML161 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7683 mL | 13.8416 mL | 27.6832 mL | 55.3664 mL | 69.208 mL |
| 5 mM | 0.5537 mL | 2.7683 mL | 5.5366 mL | 11.0733 mL | 13.8416 mL |
| 10 mM | 0.2768 mL | 1.3842 mL | 2.7683 mL | 5.5366 mL | 6.9208 mL |
| 50 mM | 0.0554 mL | 0.2768 mL | 0.5537 mL | 1.1073 mL | 1.3842 mL |
| 100 mM | 0.0277 mL | 0.1384 mL | 0.2768 mL | 0.5537 mL | 0.6921 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ML161 is a selective inhibitor of PAR1 with IC50 value of 0.26 μM [1].
Protease-activated receptor-1(PAR-1) is a unique G-protein-coupled receptor belonging to the protease-activated receptor family and its activation leads to downstream signaling events that launch a variety of cellular responses related to tumor progression [1].
ML161 is a potent PAR1 inhibitor and has a different selectivity with the reported ALK inhibitor crizotinib. When tested with human platelets, ML161 treatment inhibited the activation of thrombin-induced platelet in a dose-dependent manner by detecting P-selectin expression [1]. When tested with granule secretion, ML161 exhibited a potent inhibition on P-selectin expression in a dose-dependent manner with EC50 value of 0.3μM and also inhibited SFLLRN-induced thrombus formation [2].
References:
[1]. Dockendorff, C., et al., Discovery of 1,3-Diaminobenzenes as Selective Inhibitors of Platelet Activation at the PAR1 Receptor. ACS Med Chem Lett, 2012. 3(3): p. 232-237.
[2]. VerPlank, L., et al., Chemical Genetic Analysis of Platelet Granule Secretion-Probe 3, in Probe Reports from the NIH Molecular Libraries Program. 2010, National Center for Biotechnology Information (US): Bethesda (MD).
- Buxtamine
Catalog No.:BCC8135
CAS No.:4236-73-1
- (-)-Gallocatechin gallate
Catalog No.:BCN6328
CAS No.:4233-96-9
- SC 57461A
Catalog No.:BCC2348
CAS No.:423169-68-0
- PYZD-4409
Catalog No.:BCC4253
CAS No.:423148-78-1
- N-(4-Cyanophenyl)glycine
Catalog No.:BCC9057
CAS No.:42288-26-6
- Hesperadin
Catalog No.:BCC2174
CAS No.:422513-13-1
- Triacetyl Resveratrol
Catalog No.:BCC6482
CAS No.:42206-94-0
- DOI hydrochloride
Catalog No.:BCC5925
CAS No.:42203-78-1
- Cephalocyclidin A
Catalog No.:BCN5481
CAS No.:421583-14-4
- Artemyriantholide D
Catalog No.:BCN7478
CAS No.:421558-76-1
- N,N-Bis(2-chloroethyl)-p-toluenesulphonamide
Catalog No.:BCC9060
CAS No.:42137-88-2
- Tetradehydropodophyllotoxin
Catalog No.:BCN8395
CAS No.:42123-27-3
- 1-Benzylimidazole
Catalog No.:BCC8462
CAS No.:4238-71-5
- Pashanone
Catalog No.:BCN5482
CAS No.:42438-78-8
- Pinostilbene
Catalog No.:BCN5483
CAS No.:42438-89-1
- H-ß-Ala-OEt.HCl
Catalog No.:BCC2852
CAS No.:4244-84-2
- Flunixin Meglumin
Catalog No.:BCC4429
CAS No.:42461-84-7
- Isobutyl 4-Hydroxybenzoate
Catalog No.:BCN8412
CAS No.:4247-02-3
- 23-Hydroxylongispinogenin
Catalog No.:BCN7830
CAS No.:42483-24-9
- IPA-3
Catalog No.:BCC4978
CAS No.:42521-82-4
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Crocin
Catalog No.:BCN2373
CAS No.:42553-65-1
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- 8-Hydroxy-5-O-beta-D-glucopyranosylpsoralen
Catalog No.:BCN1443
CAS No.:425680-98-4
Identification of QTLs associated with callogenesis and embryogenesis in oil palm using genetic linkage maps improved with SSR markers.[Pubmed:23382832]
PLoS One. 2013;8(1):e53076.
Clonal reproduction of oil palm by means of tissue culture is a very inefficient process. Tissue culturability is known to be genotype dependent with some genotypes being more amenable to tissue culture than others. In this study, genetic linkage maps enriched with simple sequence repeat (SSR) markers were developed for dura (ENL48) and pisifera (ML161), the two fruit forms of oil palm, Elaeis guineensis. The SSR markers were mapped onto earlier reported parental maps based on amplified fragment length polymorphism (AFLP) and restriction fragment length polymorphism (RFLP) markers. The new linkage map of ENL48 contains 148 markers (33 AFLPs, 38 RFLPs and 77 SSRs) in 23 linkage groups (LGs), covering a total map length of 798.0 cM. The ML161 map contains 240 markers (50 AFLPs, 71 RFLPs and 119 SSRs) in 24 LGs covering a total of 1,328.1 cM. Using the improved maps, two quantitative trait loci (QTLs) associated with tissue culturability were identified each for callusing rate and embryogenesis rate. A QTL for callogenesis was identified in LGD4b of ENL48 and explained 17.5% of the phenotypic variation. For embryogenesis rate, a QTL was detected on LGP16b in ML161 and explained 20.1% of the variation. This study is the first attempt to identify QTL associated with tissue culture amenity in oil palm which is an important step towards understanding the molecular processes underlying clonal regeneration of oil palm.
Discovery of 1,3-Diaminobenzenes as Selective Inhibitors of Platelet Activation at the PAR1 Receptor.[Pubmed:22408714]
ACS Med Chem Lett. 2012 Mar 8;3(3):232-237.
A high-throughput screen of the NIH-MLSMR compound collection, along with a series of secondary assays to identify potential targets of hit compounds, previously identified a 1,3-diaminobenzene scaffold that targets protease-activated receptor 1 (PAR1). We now report additional structure-activity relationship (SAR) studies that delineate the requirements for activity at PAR1 and identify plasma-stable analogues with nanomolar inhibition of PAR1-mediated platelet activation. Compound 4 was declared as a probe (ML161) with the NIH Molecular Libraries Program. This compound inhibited platelet aggregation induced by a PAR1 peptide agonist or by thrombin but not by several other platelet agonists. Initial studies suggest that ML161 is an allosteric inhibitor of PAR1. These findings may be important for the discovery of antithrombotics with an improved safety profile.


