Sotrastaurin (AEB071)PKC inhibitor CAS# 425637-18-9 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 425637-18-9 | SDF | Download SDF |
| PubChem ID | 10296883 | Appearance | A solid |
| Formula | C25H22N6O2 | M.Wt | 438.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AEB071 | ||
| Solubility | DMSO : ≥ 50 mg/mL (114.03 mM) *"≥" means soluble, but saturation unknown. | ||
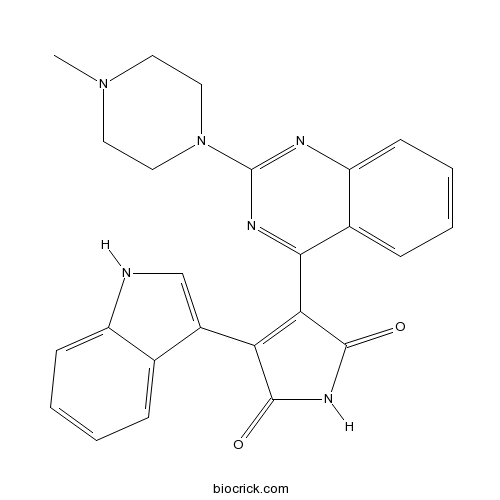
| Chemical Name | 3-(1H-indol-3-yl)-4-[2-(4-methylpiperazin-1-yl)quinazolin-4-yl]pyrrole-2,5-dione | ||
| SMILES | CN1CCN(CC1)C2=NC3=CC=CC=C3C(=N2)C4=C(C(=O)NC4=O)C5=CNC6=CC=CC=C65 | ||
| Standard InChIKey | OAVGBZOFDPFGPJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sotrastaurin is a potent and selective small-molecule inhibitor of protein kinase C (PKC) with Ki value of 0.22 nM for PKCθ. | |||||
| Targets | PKCθ | |||||
| IC50 | 0.22 nM | |||||

Sotrastaurin (AEB071) Dilution Calculator

Sotrastaurin (AEB071) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2806 mL | 11.403 mL | 22.8061 mL | 45.6121 mL | 57.0151 mL |
| 5 mM | 0.4561 mL | 2.2806 mL | 4.5612 mL | 9.1224 mL | 11.403 mL |
| 10 mM | 0.2281 mL | 1.1403 mL | 2.2806 mL | 4.5612 mL | 5.7015 mL |
| 50 mM | 0.0456 mL | 0.2281 mL | 0.4561 mL | 0.9122 mL | 1.1403 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.2281 mL | 0.4561 mL | 0.5702 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AEB071 is an inhibitor of protein kinase C (PKC). The PKC inhibitor which can block the T-cell activation has the ability of immune suppression [1].
The protein kinase C (PKC) isoforms is very important in cell signaling, proliferation, differentiation, migration, survival, and death. PKC family has many isoforms. Among the PKC isoforms, PKC isoforms have basal effect on the T cells’ activation and other immune cell functions [2,3].
ABE071 is a potent inhibitor of novel and classical PKC isoforms. Through the inhibition of PKC, AEB071 can depress the activation and proliferation of T-cell and decrease the production of cytokine.ABE071 can also suppress the NK cell activity. Ex vivo stimulation of lymphocytes from subjects exposed to single doses of AEB071 resulted in a dose-dependent inhibition of both lymphocyte proliferation and IL2 mRNA expression
AEB071 is an effective treatment strategy for the cure of autoimmune diseases. According to the Psoriasis Area Severity Index (PASI) score, after 2 weeks’ treatment with 300 mg bid AEB071, Clinical severity of psoriasis was reduced up to 69% compared with baseline[2,3].
References:
[1]. Weckbecker G1, Pally C, Beerli C, et al. Effects of the novel protein kinase C inhibitor AEB071 (Sotrastaurin) on rat cardiac allograft survival using single agent treatment or combination therapy with cyclosporine, everolimus or FTY720. Transpl Int. 2010 May 1;23(5):543-52
[2]. Skvara H1, Dawid M, Kleyn E, Wolff B, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008 Sep;118(9):3151-9.
[3]. Matz M1, Weber U, Mashreghi MF, et al. Effects of the new immunosuppressive agent AEB071 on human immune cells. Nephrol Dial Transplant. 2010 Jul;25(7):2159-67
- Crocin
Catalog No.:BCN2373
CAS No.:42553-65-1
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- IPA-3
Catalog No.:BCC4978
CAS No.:42521-82-4
- 23-Hydroxylongispinogenin
Catalog No.:BCN7830
CAS No.:42483-24-9
- Isobutyl 4-Hydroxybenzoate
Catalog No.:BCN8412
CAS No.:4247-02-3
- Flunixin Meglumin
Catalog No.:BCC4429
CAS No.:42461-84-7
- H-ß-Ala-OEt.HCl
Catalog No.:BCC2852
CAS No.:4244-84-2
- Pinostilbene
Catalog No.:BCN5483
CAS No.:42438-89-1
- Pashanone
Catalog No.:BCN5482
CAS No.:42438-78-8
- 1-Benzylimidazole
Catalog No.:BCC8462
CAS No.:4238-71-5
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
- Buxtamine
Catalog No.:BCC8135
CAS No.:4236-73-1
- 8-Hydroxy-5-O-beta-D-glucopyranosylpsoralen
Catalog No.:BCN1443
CAS No.:425680-98-4
- Luteolin-6-C-glucoside
Catalog No.:BCN4985
CAS No.:4261-42-1
- TAK-700 (Orteronel)
Catalog No.:BCC2280
CAS No.:426219-18-3
- TAK-700 salt
Catalog No.:BCC1979
CAS No.:426219-53-6
- 21,23-Dihydro-23-hydroxy-21-oxozapoterin
Catalog No.:BCN7230
CAS No.:426266-88-8
- Dehydrodiconiferyl alcohol
Catalog No.:BCN6878
CAS No.:4263-87-0
- Hemapolin
Catalog No.:BCC8994
CAS No.:4267-80-5
- 4-Hydroxy-3-methoxyphenyl O-beta-D-6-O-syringate-glucopyranoside
Catalog No.:BCN1442
CAS No.:426821-85-4
- NS 5806
Catalog No.:BCC7872
CAS No.:426834-69-7
- S-Isopropylisothiourea hydrobromide
Catalog No.:BCC6837
CAS No.:4269-97-0
- Cyproterone Acetate
Catalog No.:BCC3758
CAS No.:427-51-0
- Pedunculoside
Catalog No.:BCN1191
CAS No.:42719-32-4
Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production.[Pubmed:24192715]
J Invest Dermatol. 2014 Apr;134(4):975-983.
Regulatory T-cells (Treg) are crucial for immune homeostasis and prevention of immune pathology. Yet, Treg may lose Foxp3 and start secreting IL-17, dependent on environmental cues. Our previous data revealed that Treg from severe psoriasis patients are particularly prone to such conversion. The question of how to maintain Treg stability in the context of inflammation awaits immediate resolution. The pan-protein kinase C (PKC) inhibitor sotrastaurin has shown efficacy in clinical trials of psoriasis. Here, we show that sotrastaurin inhibited effector T-cell responses, whereas the regulatory response was enhanced. Sotrastaurin prevented TCR/CD28-induced T-cell activation and pro-inflammatory cytokine production, but preserved a stable Treg phenotype as evidenced by maintenance of suppressive capacity, high Foxp3 and CD25 expression, and lack of IL-17A and IFNgamma production. Moreover, in both circulating and dermal psoriatic Treg, prone to rapid induction of IL-17, sotrastaurin enhanced Foxp3 expression and prevented IL-17A and IFNgamma production even when stimulated in the presence of the helper T 17-enhancing cytokines IL-1beta or IL-23. Thus, pharmacological inhibition of PKC may serve as a powerful tool to concurrently inhibit effector T cells and to facilitate Treg, thereby showing therapeutic potential for the treatment of psoriasis.
AEB071 (sotrastaurin) does not exhibit toxic effects on human islets in vitro, nor after transplantation into immunodeficient mice.[Pubmed:21934354]
Islets. 2011 Nov-Dec;3(6):338-43.
AEB071 (AEB, sotrastaurin), a specific inhibitor of protein kinase C, reduces T-lymphocyte activation and cytokine release. AEB delays islet allograft rejection in rats and prevents rejection when combined with cyclosporine. Since many immunosuppressive agents have toxic effects on the function of transplanted islets, we investigated whether this was also the case with AEB. Human islets were transplanted into Rag-knockout mice randomly assigned to vehicle control, AEB or sirolimus treatment groups. Non-fasting blood glucose levels, body weight and glucose tolerance was measured in recipients. In a separate experiment, human islets were cultured in the presence of AEB and assayed for glucose dependent insulin secretion and level of beta-cell apoptosis. Eighty-six percent of the AEB-treated recipients achieved normoglycemia following transplant (compared with none in sirolimus-treated group, p < 0.05). AEB-treated recipients exhibited similar glucose homeostasis as vehicle-treated controls, which was better than in sirolimus-treated recipients. Human islets cultured with AEB showed similar rates of beta-cell apoptosis (p = 0.98 by one-way ANOVA) and glucose stimulated insulin secretion (p = 0.15) as those cultured with vehicle. These results suggest that AEB is not associated with toxic effects on islet engraftment or function. AEB appears to be an appropriate immunosuppressive candidate for clinical trials in islet transplantation.
Sotrastaurin (AEB071) alone and in combination with cyclosporine A prolongs survival times of non-human primate recipients of life-supporting kidney allografts.[Pubmed:22179400]
Transplantation. 2012 Jan 27;93(2):156-64.
BACKGROUND: Sotrastaurin (STN), a novel oral protein kinase C inhibitor that inhibits early T-cell activation, was assessed in non-human primate recipients of life-supporting kidney allografts. METHODS: Cynomolgus monkey recipients of life-supporting kidney allografts were treated orally with STN alone or in combination with cyclosporine A (CsA). RESULTS: STN monotherapy at 50 mg/kg once daily prolonged recipient survival times to the predefined endpoint of 29 days (n=2); when given at 25 mg/kg twice daily, the median survival time (MST) was 27 days (n=4). Neither once-daily monotherapy of STN 20 mg/kg nor CsA 20 mg/kg was effective (MST 6 days [n=2] and 7 days [n=5], respectively). In combination, however, STN 20 mg/kg and CsA 20 mg/kg prolonged MST to more than 100 days (n=5). By combining lower once-daily doses of STN (7 or 2 mg/kg) with CsA (20 mg/kg), MST was more than 100 (n=3) and 22 days (n=2), respectively. Neither in single-dose pharmacokinetic studies nor the transplant recipients were STN or CsA blood levels for combined treatment greater than when either drug was administered alone. STN blood levels in transplant recipients during combination therapy were dose related (20 mg/kg, 30-182 ng/mL; 7 mg/kg, 7-41 ng/mL; and 2 mg/kg, 3-5 ng/mL). STN at a daily dose of up to 20 mg/kg was relatively well tolerated. CONCLUSIONS: STN prolonged survival times of non-human primate kidney allograft recipients both as monotherapy and most effectively in combination with CsA. Pharmacokinetic interactions were not responsible for the potentiation of immunosuppressive efficacy by coadministering STN and CsA.
The protein kinase C inhibitor AEB071 (sotrastaurin) modulates migration and superoxide anion production by human neutrophils in vitro.[Pubmed:23058012]
Int J Immunopathol Pharmacol. 2012 Jul-Sep;25(3):617-26.
We examined the effect of the protein kinase C-selective inhibitor AEB071 (sotrastaurin) on neutrophil functions in vitro. Pre-incubation with AEB071 at concentrations similar to those reached during in vivo therapy significantly reduced cell capacity to migrate toward three different chemo-attractants and to produce superoxide anions (O(2)(-)) in response to phorbol myristate acetate (PMA) or to N-formyl-methionyl-leucyl-phenylalanine (fMLP). AEB071 also significantly inhibited the O(2)(-) overproduction induced by fMLP in neutrophils primed with tumor necrosis factor alpha (TNF-alpha) or granulocyte/macrophage-colony stimulating factor (GM-CSF). This inhibition was not linked to fMLP-receptor down-regulation since the drug had no effect on either fMLP-receptors or fMLP-induced CD11b membrane expression. When the activity of AEB071 was compared to that of the conventional protein kinase C (PKC) inhibitor Go6850 (which, like sotrastaurin, inhibits classical and novel PKC isoforms), Go6976 (an inhibitor of alpha and alpha PKC isoforms) and rottlerin (a prevailing delta PKC isoform inhibitor), AEB071 at an equimolar concentration of 3 muM (close to the maximum drug concentration reached in patients treated with AEB071) caused significantly more inhibition on both chemotactic response and superoxide production. These in vitro findings suggest that neutrophils may offer a cellular target for AEB071 activity in vivo.


