Enzastaurin (LY317615)PKC beta inhibitor,potent and selective CAS# 170364-57-5 |
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 170364-57-5 | SDF | Download SDF |
| PubChem ID | 176167 | Appearance | Powder |
| Formula | C32H29N5O2 | M.Wt | 515.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LY317615;enzastaurin | ||
| Solubility | DMSO : 8.33 mg/mL (16.16 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
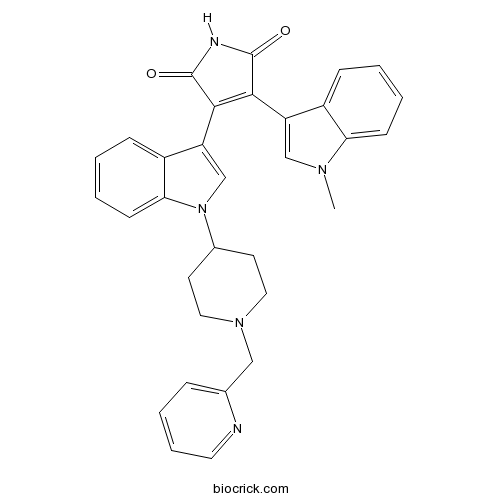
| Chemical Name | 3-(1-methylindol-3-yl)-4-[1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]indol-3-yl]pyrrole-2,5-dione | ||
| SMILES | CN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=CN(C5=CC=CC=C54)C6CCN(CC6)CC7=CC=CC=N7 | ||
| Standard InChIKey | AXRCEOKUDYDWLF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C32H29N5O2/c1-35-19-25(23-9-2-4-11-27(23)35)29-30(32(39)34-31(29)38)26-20-37(28-12-5-3-10-24(26)28)22-13-16-36(17-14-22)18-21-8-6-7-15-33-21/h2-12,15,19-20,22H,13-14,16-18H2,1H3,(H,34,38,39) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent PKCβ inhibitor (IC50 values are 6, 39, 83 and 110 nM for PKCβ, PKCα, PKCγ and PKCε, respectively). Suppresses proliferation and induces apoptosis in HCT116 colon carcinoma and U87MG glioblastoma cells in vitro. Reduces tumor growth and exhibits antiangiogenic activity in HT-29 colon carcinoma xenografts in mice. Also suppresses growth of HCT116 and U87MG xenografts in mice. Orally active. |

Enzastaurin (LY317615) Dilution Calculator

Enzastaurin (LY317615) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9395 mL | 9.6973 mL | 19.3945 mL | 38.789 mL | 48.4863 mL |
| 5 mM | 0.3879 mL | 1.9395 mL | 3.8789 mL | 7.7578 mL | 9.6973 mL |
| 10 mM | 0.1939 mL | 0.9697 mL | 1.9395 mL | 3.8789 mL | 4.8486 mL |
| 50 mM | 0.0388 mL | 0.1939 mL | 0.3879 mL | 0.7758 mL | 0.9697 mL |
| 100 mM | 0.0194 mL | 0.097 mL | 0.1939 mL | 0.3879 mL | 0.4849 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The efficacy of docetaxel/prednisone with or without enzastaurin, an inhibitor of the beta isoform of protein kinase C with therapeutic activity against prostate cancer, in patients with castration-resistant metastatic prostate cancer has been explored.
Abstract
Follicular lymphoma cells are characterized not only by genetic alterations involving Bcl-2, Bcl-6 OR C-mYc but also by alterations in B-cell receptor signaling pathways.
Abstract
PFS-6 was evaluated in newly diagnosed gliobstoma patients who were MGMT- promoter-hypermethylation-free and posturgically treated with enzastaurin plus radiation.
Abstract
The combination of enzastaurin and bevacizumab, which showed synergistic antitumor activity in preclinical studies, was evaluated for safety and efficacy.
Abstract
Enzastaurin with 5-fluorouracil/leucovorin plus bevacizumab was evaluated as maintance therapy for MCRC, since the combination of enzastaurin and bevacizumab was well-tolerated and demonstrated synergistic antitumor effects in phse 1 studies.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Enzastaurin is an ATP-competitive, selective oral inhibitor of protein kinase Cβ with IC50 value of 6 nM [1].
Protein kinase C (PKC) is a family of serine-threonine protein kinases that have been proved to play critical roles in the formation and progression of tumor cells. The PKCβ is especially found to contribute to the growth and proliferation of tumors such as diffuse large B-cell lymphoma, multiple myeloma and chronic lymphoid leukemia. The selective PKCβ inhibitor enzastaurin was found to have antiangiogenic activity in tumor model as well as suppress tumor proliferation and induce apoptosis. Besides that, enzastaurin showed the inhibitory effects on phosphorylation of ribosomal protein S6, GSK3β and AKT which are in pathways influenced by PKC. Due to these, enzastaurin was developed as a therapy for cancer [1 and 2].
Enzastaurin at low concentration suppressed cell proliferation of various tumor cells including colon carcinoma (HCT-116), glioblastoma (U87MG), non–small cell lung cancer (A549), melanoma (M14), ovarian cancer (OVCAR-3), breast cancer (MCF-7), leukemia (K562), prostate cancer (PC-3), renal cancer (CAKI-1) and central nervous system cancer (U251). Enzastaurin induced apoptosis of tumor cells at low concentration in a range of 1 to 4 μM and the apoptosis was proved to be caspase-independent. In addition, enzastaurin suppressed the phosphorylation ofGSK3βSer9, ribosomal protein S6Ser240/244 and AKTThr308 time-dependently in tumor cells and affected these pathways [1 and 3].
In mice models, oral administration of enzastaurin at a dose of 75 mg/kg resulted in significant proliferation inhibition of U87MG glioblastoma or HCT116 coloncarcinoma xenografts. In mice bearing human walden strommacro globulinemia xenografts, administration of enzastaurin at dose of 80 mg/kg significantly reduced tumor growth of WM and increased survival [1 and 2].
References:
[1] Graff J R, McNulty A M, Hanna K R, et al. The protein kinase Cβ–selective inhibitor, enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Research, 2005, 65(16): 7462-7469.
[2] Moreau A S, Jia X, Ngo H T, et al. Protein kinase C inhibitor enzastaurin induces in vitro and in vivo antitumor activity in Waldenstr?mmacroglobulinemia. Blood, 2007, 109(11): 4964-4972.
[3] Rizvi M A, Ghias K, Davies K M, et al. Enzastaurin (LY317615), a protein kinase Cβ inhibitor, inhibits the AKT pathway and induces apoptosis in multiple myeloma cell lines. Molecular cancer therapeutics, 2006, 5(7): 1783-1789.
- CD 2665
Catalog No.:BCC7778
CAS No.:170355-78-9
- CD 2314
Catalog No.:BCC6071
CAS No.:170355-37-0
- 24-Methylenecycloartane-3beta,26-diol
Catalog No.:BCN1530
CAS No.:17020-27-8
- Bauerenol acetate
Catalog No.:BCN1106
CAS No.:17020-04-1
- 11-Keto-beta-boswellic acid
Catalog No.:BCN2298
CAS No.:17019-92-0
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- Curcolone
Catalog No.:BCN3559
CAS No.:17015-43-9
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- Pseudobufarenogin
Catalog No.:BCN8234
CAS No.:17008-69-4
- Bufarenogin
Catalog No.:BCN2297
CAS No.:17008-65-0
- Reserpine hydrochloride
Catalog No.:BCC4279
CAS No.:16994-56-2
- LY 333531 hydrochloride
Catalog No.:BCC7969
CAS No.:169939-93-9
- 4-(6-Methyl-4-oxohept-5-en-2-yl)cyclohex-2-en-1-one
Catalog No.:BCN7528
CAS No.:170380-68-4
- 1,4-Epidioxybisabola-2,10-dien-9-one
Catalog No.:BCN7532
CAS No.:170380-69-5
- Isohyperectine
Catalog No.:BCN3405
CAS No.:170384-75-5
- SC 236
Catalog No.:BCC7809
CAS No.:170569-86-5
- Oteromycin
Catalog No.:BCN1849
CAS No.:170591-45-4
- YC 1
Catalog No.:BCC7912
CAS No.:170632-47-0
- Fmoc-D-Abu-OH
Catalog No.:BCC3203
CAS No.:170642-27-0
- α-Conotoxin EI
Catalog No.:BCC5979
CAS No.:170663-33-9
- D-Mannitol diacetonide
Catalog No.:BCC8951
CAS No.:1707-77-3
- Bindone
Catalog No.:BCC8877
CAS No.:1707-95-5
- 6beta-Hydroxyhispanone
Catalog No.:BCN7453
CAS No.:170711-93-0
- Nociceptin
Catalog No.:BCC5686
CAS No.:170713-75-4
A phase II trial of enzastaurin (LY317615) in combination with bevacizumab in adults with recurrent malignant gliomas.[Pubmed:26643807]
J Neurooncol. 2016 Mar;127(1):127-35.
We evaluated the efficacy of combination Enzastaurin (LY317615) and bevacizumab for recurrent malignant gliomas and explored serologic correlates. We enrolled 81 patients with glioblastomas (GBM, n = 40) and anaplastic gliomas (AG, n = 41). Patients received enzastaurin as a loading dose of 1125 mg, followed by 500 or 875 mg daily for patients on non-enzyme-inducing or enzyme-inducing antiepileptics, respectively. Patients received bevacizumab 10 mg/kg intravenously biweekly. Clinical evaluations were repeated every 4 weeks. Magnetic resonance imaging was obtained at baseline and every 8 weeks from treatment onset. Phosphorylated glycogen synthase kinase (GSK)-3 levels from peripheral blood mononuclear cells (PBMCs) were checked with each MRI. Median overall survival was 7.5 and 12.4 months for glioblastomas and anaplastic glioma cohorts, with median progression-free survivals of 2.0 and 4.4 months, respectively. Of GBM patients, 3/40 (7.5 %) were not evaluable, while 8/37 (22 %) had partial or complete response and 20/37 (54 %) had stable disease for 2+ months. Of the 39 evaluable AG patients, 18 (46 %) had an objective response, and 16 (41 %) had stable disease for 2+ months. The most common grade 3+ toxicities were lymphopenia (15 %), hypophosphatemia (8.8 %) and thrombotic events (7.5 %). Two (2.5 %) GBM patients died suddenly; another death (1.3 %) occurred from intractable seizures. Phosphorylated GSK-3 levels from PBMCs did not correlate with treatment response. A minimally important improvement in health-related quality of life was self-reported in 7-9/24 (29.2-37.5 %). Early response based on Levin criteria was significantly associated with significantly longer progression free survival for glioblastomas. Enzastaurin (LY317615) in combination with bevacizumab for recurrent malignant gliomas is well-tolerated, with response and progression-free survival similar to bevacizumab monotherapy.
Phase I dose escalation and pharmacokinetic study of oral enzastaurin (LY317615) in advanced solid tumors.[Pubmed:20707806]
Cancer Sci. 2010 Oct;101(10):2193-9.
Enzastaurin is an oral serine/threonine kinase inhibitor that targets the protein kinase C (PKC) and phosphoinositide 3-kinase/AKT pathways to induce apoptosis and suppress proliferation of various cancer cell lines. This phase I study evaluated the tolerability and pharmacokinetics of enzastaurin in Japanese patients with advanced solid tumors and determined the recommended dose for phase II. Eligible patients had advanced solid tumors and an Eastern Cooperative Oncology Group performance status of 0-2. Patients received enzastaurin orally once daily until disease progression (PD) or unacceptable toxicity occurred. Enzastaurin was started at 250 mg/day followed by stepwise dose increases based on the incidence of dose-limiting toxicities (DLT). Twenty-three patients (seven patients: 250 mg; six patients: 375 mg; six patients: 500 mg; four patients: 750 mg) were enrolled and received enzastaurin. The major tumor types were non-small-cell lung cancer (n = 5) and breast cancer (n = 3). No DLT was reported at doses of 500 mg or less. Because two DLT (grade 2 QTc prolongation lasting for a week) were observed at 750 mg enzastaurin, this was determined as the maximum tolerated dose. Multiple daily doses at 500 mg achieved the target plasma concentration to inhibit PKC activity (1400 nmol/L). Enzastaurin was well tolerated up to 500 mg in Japanese patients with advanced solid tumors. The recommended dose for phase II was determined to be 500 mg daily for a 28-day cycle on the basis of safety and plasma exposures.
Phase 2 randomized study of enzastaurin (LY317615) for lung cancer prevention in former smokers.[Pubmed:23065656]
Cancer. 2013 Mar 1;119(5):1023-32.
BACKGROUND: Chemoprevention for lung cancer with nutraceutical or anti-inflammatory agents has had mixed clinical benefit. Novel targeted agents hold the promise of greater efficacy and selectivity. The authors of this report evaluated enzastaurin, a selective protein kinase C-beta (PKC-beta) inhibitor with antiproliferative and proapoptotic properties, in former smokers. METHODS: The primary objective of this study was to compare the average fraction of Ki-67-stained cells (the Ki-67 labeling index [LI]) in bronchial biopsy specimens that were collected before and after treatment. Participants were randomized (2:1) to receive either 6 months of daily oral enzastaurin (500 mg) or placebo. Stratification was based on morphology, history of lung cancer, and airway obstruction. RESULTS: In pretrial investigations, the rationale for PKC-beta inhibition and pathway interrogation was established in premalignant lesions and early stage lung cancer. In an intent-to-treat analysis, of 40 randomized participants, there was no significant difference in the pretreatment/post-treatment change in the Ki-67 LI between the enzastaurin group and the placebo group (P = .53). Six participants discontinued enzastaurin, including 4 participants who had adverse events, including abdominal distension, deep vein thrombosis, hyponatremia, and rash, and 2 participants who decided to discontinue. One participant in the placebo group was discontinued on the study because of noncompliance. Two participants had >/=1 serious adverse event (bradycardia, deep vein thrombosis, and hypotension). CONCLUSIONS: To the authors' knowledge, this represents the first chemoprevention trial with a non-US Food and Drug Administration-approved, oral, small-molecule-targeted agent. Although the primary endpoint was not met, enzastaurin was tolerable for 6 months by 75% of participants, and there was a suggestion of response in a subset analysis that was restricted to those who had metaplastic or dysplastic lesions.
A phase I study of LY317615 (enzastaurin) and temozolomide in patients with gliomas (EORTC trial 26054).[Pubmed:22291006]
Neuro Oncol. 2012 Mar;14(3):344-50.
We report a phase 1 study to examine the safety and recommended dose of the oral protein kinase C-beta inhibitor (anti-angiogenic) enzastaurin in combination with single-agent temozolomide. The study was conducted in patients with recurrent glioblastoma or newly diagnosed disease that was not treatable with standard (chemo)radiotherapy. Patients were treated with standard dose temozolomide (200 mg/m(2) for 5 days every 4 weeks) together with daily oral enzastaurin. Three dose levels of enzastaurin were investigated: 250 mg daily (OD), 500 mg OD, and 250 mg twice daily (BID). Dose-limiting toxicity was determined in the first 2 cycles, but treatment continued until limiting toxicity or disease progression was identified. Twenty-eight patients were enrolled. No dose-limiting toxicity was noted at 250 mg OD or 500 mg OD. However, at 250 mg BID, 2 dose-limiting episodes of thrombocytopenia were noted. The recommended dose for enzastaurin in combination with standard 4-weekly temozolomide is therefore 500 mg OD. The pharmacokinetics of enzastaurin in combination with temozolomide was evaluated. Temozolomide did not appear to effect enzastaurin exposures at the 250 mg or 500 mg OD dose levels.
The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts.[Pubmed:16103100]
Cancer Res. 2005 Aug 15;65(16):7462-9.
Activation of protein kinase Cbeta (PKCbeta) has been repeatedly implicated in tumor-induced angiogenesis. The PKCbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses angiogenesis and was advanced for clinical development based upon this antiangiogenic activity. Activation of PKCbeta has now also been implicated in tumor cell proliferation, apoptosis, and tumor invasiveness. Herein, we show that Enzastaurin has a direct effect on human tumor cells, inducing apoptosis and suppressing the proliferation of cultured tumor cells. Enzastaurin treatment also suppresses the phosphorylation of GSK3betaser9, ribosomal protein S6(S240/244), and AKT(Thr308). Oral dosing with Enzastaurin to yield plasma concentrations similar to those achieved in clinical trials significantly suppresses the growth of human glioblastoma and colon carcinoma xenografts. As in cultured tumor cells, Enzastaurin treatment suppresses the phosphorylation of GSK3beta in these xenograft tumor tissues. Enzastaurin treatment also suppresses GSK3beta phosphorylation to a similar extent in peripheral blood mononuclear cells (PBMCs) from these treated mice. These data show that Enzastaurin has a direct antitumor effect and that Enzastaurin treatment suppresses GSK3beta phosphorylation in both tumor tissue and in PBMCs, suggesting that GSK3beta phosphorylation may serve as a reliable pharmacodynamic marker for Enzastaurin activity. With previously published reports, these data support the notion that Enzastaurin suppresses tumor growth through multiple mechanisms: direct suppression of tumor cell proliferation and the induction of tumor cell death coupled to the indirect effect of suppressing tumor-induced angiogenesis.
Antiangiogenic and antitumor effects of a protein kinase Cbeta inhibitor in human HT-29 colon carcinoma and human CaKi1 renal cell carcinoma xenografts.[Pubmed:11848470]
Anticancer Res. 2001 Sep-Oct;21(5):3175-84.
The compound 317615 x 2HCl, a selective protein kinase Cbeta inhibitor, was not very cytotoxic toward human CaKi1 renal cell carcinoma cells or human HT-29 colon carcinoma cells in monolayer culture. Isobologram analysis was used to determine additivity or synergy of the combination regimens. Exposure of CaKi1 cells to 317615 x 2HCl (10 or 100 mM) along with gemcitabine or 5-fluorouracil for 24 hours resulted in cytotoxicity that appeared to be less-than-additive to additive for the two agents. Exposure of HT-29 cells to gemcitabine along with 317615 x 2HCl (10 mM or 100 mM) resulted in a synergistic cytotoxicity while combinations with 5-fluorouracil resulted in additive to greater-than-additive cytotoxicity for the agents. After treatment of CaKi1 or HT-29 xenograft-bearing mice with 317615 x 2HCl, immunohistochemical staining for expression of endothelial specific markers, either CD31 or CD105, was used to quantify the number of intratumoral vessels in the samples. CaKi1 tumor angiogenesis was very responsive to treatment with 317615 x 2HCl such that the number of intratumoral vessels stained by CD31 or CD105 was decreased to 20% of the control. The HT-29 colon carcinoma angiogenesis was also responsive to 317615 x 2HCl, such that the number of intratumoral vessels stained by CD31 or CD105 was decreased to 40% to 50% of the controL 5-fluorouracil, cisplatin or fractionated radiation therapy was combined with treatment with 317615 x 2HCl in the simultaneous combination treatment regimen in animals bearing HT-29 colon carcinoma xenografts. The resulting tumor growth delays indicated that administration of 317615 x 2HCl increased the effects of the cytotoxic therapy. Both a simultaneous or an overlapping treatment regimen and a sequential treatment regimen were used to assess 317615 x 2HCl alone and along with fractionated radiation therapy or gemcitabine against the human CaKi1 renal cell carcinoma xenograft. The CaKi1 tumor was quite sensitive to fractionated radiation therapy and to gemcitabine and, although 317615 x 2HCl was an effective single agent in this tumor, the combination regimens did not reach additivity for the combination regimens in vivo. 317615 x 2HCl is in early clinical testing.


