Go 6983pan-PKC inhibitor CAS# 133053-19-7 |
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- K-252c
Catalog No.:BCC3706
CAS No.:85753-43-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 133053-19-7 | SDF | Download SDF |
| PubChem ID | 3499 | Appearance | Powder |
| Formula | C26H26N4O3 | M.Wt | 442.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Goe 6983 | ||
| Solubility | DMSO : ≥ 34 mg/mL (76.83 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
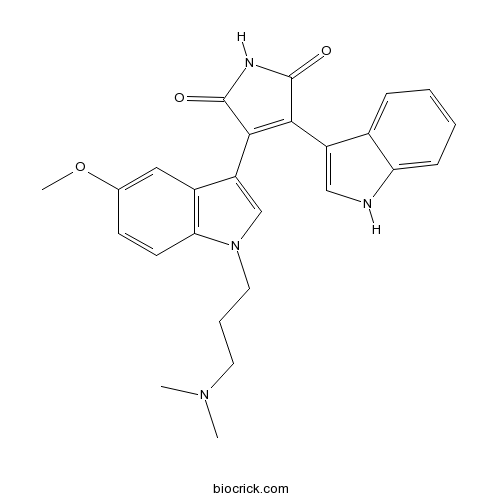
| Chemical Name | 3-[1-[3-(dimethylamino)propyl]-5-methoxyindol-3-yl]-4-(1H-indol-3-yl)pyrrole-2,5-dione | ||
| SMILES | CN(C)CCCN1C=C(C2=C1C=CC(=C2)OC)C3=C(C(=O)NC3=O)C4=CNC5=CC=CC=C54 | ||
| Standard InChIKey | LLJJDLHGZUOMQP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H26N4O3/c1-29(2)11-6-12-30-15-20(18-13-16(33-3)9-10-22(18)30)24-23(25(31)28-26(24)32)19-14-27-21-8-5-4-7-17(19)21/h4-5,7-10,13-15,27H,6,11-12H2,1-3H3,(H,28,31,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Broad spectrum protein kinase C (PKC) inhibitor (IC50 values are 7, 7, 6, 10, 60 and 20000 nM for PKCα, PKCβ, PKCγ, PKCδ, PKCζ and PKCμ respectively). Displays cardioprotective properties; reduces polymorphonuclear leukocyte adherence and infiltration following myocardial ischemia/reperfusion injury. Optimizes naïve human pluripotent stem cell growth and viability following naïve cell derivation from primed ESCs and iPSCs using naïve human stem cell medium (NHSM). |

Go 6983 Dilution Calculator

Go 6983 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2598 mL | 11.2992 mL | 22.5984 mL | 45.1967 mL | 56.4959 mL |
| 5 mM | 0.452 mL | 2.2598 mL | 4.5197 mL | 9.0393 mL | 11.2992 mL |
| 10 mM | 0.226 mL | 1.1299 mL | 2.2598 mL | 4.5197 mL | 5.6496 mL |
| 50 mM | 0.0452 mL | 0.226 mL | 0.452 mL | 0.9039 mL | 1.1299 mL |
| 100 mM | 0.0226 mL | 0.113 mL | 0.226 mL | 0.452 mL | 0.565 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Go 6983 is a selective inhibitor of PKCα, PKCβ, PKCγ, PKCδ and PKCμ with IC50 value of 7 nM, 7 nM, 6 nM, 10 nM, and 20 mM, respectively [1].
Protein kinase C (PKC) serves as the receptor for tumor-promoting phorbol esters, which are potent activators of conventional (c) and novel (n) PKCs and has several isotypes: PKCα, PKCβ, PKCγ, PKCδ, PKCη and PKCμ. PKCη is reported progressively increased in MCF-10A (non-tumorigenic breast cancer cells) and promotes breast cancer cell survival [2, 3].
Go 6983 is a potent PKC inhibitor and has a different mechanism with the reported PKC inhibitor Ro-317549. When tested with MCF-7 and T47D cells, Go 6983 showed reversible effect on the down-regulation of PKCαand PKCδ induced by PKC activator PDBu, while decreased the expression of PKCη which up-regulated by PDBu. Further, Go 6983 treatment inhibited the MCF-7 cell survival by inhibiting the expression of PKCη [2]. In ARCcPE cells, PMA treatment promoted the EMT process by up-regulating the expression of PKCs(PKCα, PKCβand PKCγ) which could be significantly inhibited by Go6983 treatment through inhibiting PKCs expression at the dose of 200 nM and completely inhibited at the dose of 1000 nM [3].
In chromaffin cells isolated from female mice, Go 6983 treatment before AP-stimulus protocol reconciled the basal capcacitance values, decreased the capacitance and reduced calcium current density in a PKC dependent manner. To note, these phenomenons were more obvious in female mice compared with male mice [4].
References:
[1]. Gschwendt, M., et al., Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett, 1996. 392(2): p. 77-80.
[2]. Pal, D., S.P. Outram, and A. Basu, Upregulation of PKCeta by PKCepsilon and PDK1 involves two distinct mechanisms and promotes breast cancer cell survival. Biochim Biophys Acta, 2013. 1830(8): p. 4040-5.
[3]. He, H., et al., Phorbol ester phorbol-12-myristate-13-acetate induces epithelial to mesenchymal transition in human prostate cancer ARCaPE cells. Prostate, 2010. 70(10): p. 1119-26.
[4]. Chan, S.A., J. Hill, and C. Smith, Reduced calcium current density in female versus male mouse adrenal chromaffin cells in situ. Cell Calcium, 2012. 52(3-4): p. 313-20.
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- LY 225910
Catalog No.:BCC6891
CAS No.:133040-77-4
- Ethacrynic acid - d5
Catalog No.:BCC7987
CAS No.:1330052-59-9
- (+)-SK&F 10047 hydrochloride
Catalog No.:BCC6928
CAS No.:133005-41-1
- 13-Epijhanol
Catalog No.:BCN4713
CAS No.:133005-15-9
- Trichlormethiazide
Catalog No.:BCC4872
CAS No.:133-67-5
- 3-Indolebutyric acid (IBA)
Catalog No.:BCC6491
CAS No.:133-32-4
- Asarinin
Catalog No.:BCN2769
CAS No.:133-05-1
- (-)-Asarinin
Catalog No.:BCN2290
CAS No.:133-04-0
- Fmoc-Lys(Fmoc)-OPfp
Catalog No.:BCC3522
CAS No.:132990-14-8
- Mequindox
Catalog No.:BCC9021
CAS No.:13297-17-1
- Macrocarpal A
Catalog No.:BCN6178
CAS No.:132951-90-7
- ZD 7288
Catalog No.:BCC6884
CAS No.:133059-99-1
- BIM 23052
Catalog No.:BCC5945
CAS No.:133073-82-2
- Fmoc-D-Lys(Boc)-OPfp
Catalog No.:BCC3527
CAS No.:133083-36-0
- Crassifoline methine
Catalog No.:BCN1793
CAS No.:133084-00-1
- 3PO
Catalog No.:BCC5616
CAS No.:13309-08-5
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Flutamide
Catalog No.:BCC4364
CAS No.:13311-84-7
- Epimedin B1
Catalog No.:BCN8199
CAS No.:133137-58-3
- GR 73632
Catalog No.:BCC5801
CAS No.:133156-06-6
- Oxypeucedan hydrate
Catalog No.:BCN8372
CAS No.:133164-11-1
- Fmoc-Cit-OH
Catalog No.:BCC3180
CAS No.:133174-15-9
PKC inhibitors RO 31-8220 and Go 6983 enhance epinephrine-induced platelet aggregation in catecholamine hypo-responsive platelets by enhancing Akt phosphorylation.[Pubmed:21345315]
BMB Rep. 2011 Feb;44(2):140-5.
Impaired responsiveness of platelets to epinephrine (epi) and other catecholamines (CA) has been reported in approximately 20% of the healthy Korean and Japanese populations. In the present study, platelet aggregation induced by epi was potentiated by RO 31-8220 (RO) or Go 6983 (Go). Phosphorylated Akt (p-Akt) was very low in epi-stimulated PRP from CA-hypo-responders (CA-HY), whereas it was detected in those from CA-good responders (CA-GR). RO and Go increased p-Akt, one of the major downstream effectors of phosphoinositol-3 kinase (PI3K), in epi-stimulated PRP from both groups. Wortmannin, a PI3K inhibitor, attenuated the RO or Go-induced potentiation of p-Akt in epi-stimulated PRP, suggesting positive effects for RO and Go on PI3K. TXA(2) formation was increased by the addition of either RO or Go in epi-stimulated platelets. The present data also suggest that impaired Akt phosphorylation may be responsible for epinephrine hypo-responsiveness of platelets.
Go 6983: a fast acting protein kinase C inhibitor that attenuates myocardial ischemia/reperfusion injury.[Pubmed:16252018]
Cardiovasc Drug Rev. 2005 Fall;23(3):255-72.
Reperfusion injury is characterized by a decrease in endothelial release of nitric oxide within 5 min after reperfusion, increased leukocyte-endothelium interaction, and transmigration of leukocytes into the myocardium, producing cardiac contractile dysfunction. Go 6983 is a fast acting, lipid soluble, broad spectrum protein kinase C inhibitor. When administered at the beginning of reperfusion, it can restore cardiac function within 5 min and attenuate the deleterious effects associated with acute ischemia/reperfusion. Go 6983 may offer greater cardioprotection than other broad-spectrum PKC inhibitors in postischemic reperfusion injury because it inhibits PKC(zeta) as well as four other isoforms. The cardioprotection is associated with decreased leukocyte superoxide release and increased endothelial derived nitric oxide from vascular tissue. In vitro studies of human tissue showed that Go 6983 significantly inhibited antigen-induced superoxide release from leukocytes of patients previously sensitized to tree pollen. In human vascular tissue, Go 6983 inhibited intracellular Ca(2+) accumulation, suggesting a mechanism for its vasodilator properties. These studies suggest that Go 6983 would be an effective compound to use in a clinical ischemia/reperfusion setting of organ transplantation and/or cerebral ischemia where inhibiting superoxide release and vasoconstriction in postischemic tissues would allow for better restoration of organ function during reperfusion. However, given the broad-spectrum action of Go 6983, careful titration of the dose regimen would be recommended to ensure a successful outcome in the setting of organ transplantation and/or cerebral ischemia.
Go 6983 exerts cardioprotective effects in myocardial ischemia/reperfusion.[Pubmed:15071351]
J Cardiovasc Pharmacol. 2004 May;43(5):645-56.
Ischemia followed by reperfusion (I/R) in the presence of polymorphonuclear leukocytes (PMNs) results in cardiac contractile dysfunction. Inhibiting protein kinase C (PKC) inhibits the release of superoxide from PMNs. The compound Go 6983 is an inhibitor of all five PKC isoforms present in PMNs. Therefore, we hypothesized that Go 6983 could attenuate PMN-induced cardiac dysfunction by suppression of superoxide production from PMNs. We studied isolated rat hearts following ischemia (20 minutes) and reperfusion (45 minutes) infused with activated PMNs. In hearts reperfused with PMNs and Go 6983 (100 nM, n = 7), left ventricular developed pressure (LVDP) and the rate of LVDP (+dP/dt max) recovered to 89 +/- 7% and 74 +/- 2% of baseline values, respectively, at 45 minutes postreperfusion compared with I/R hearts (n = 9) receiving PMNs alone, which only recovered to 55 +/- 3% and 45 +/- 5% of baseline values for LVDP and +dP/dtmax, respectively (P < 0.01). Go 6983 (100 nM) significantly reduced PMN adherence to the endothelium and infiltration into the myocardium compared with I/R + PMN hearts (P < 0.01), and significantly inhibited superoxide release from PMNs by 90 +/- 2% (P < 0.01). In the presence of PMNs, Go 6983 attenuated post-I/R cardiac contractile dysfunction, which may be related in part to decreased superoxide production.
Derivation of novel human ground state naive pluripotent stem cells.[Pubmed:24172903]
Nature. 2013 Dec 12;504(7479):282-6.
Mouse embryonic stem (ES) cells are isolated from the inner cell mass of blastocysts, and can be preserved in vitro in a naive inner-cell-mass-like configuration by providing exogenous stimulation with leukaemia inhibitory factor (LIF) and small molecule inhibition of ERK1/ERK2 and GSK3beta signalling (termed 2i/LIF conditions). Hallmarks of naive pluripotency include driving Oct4 (also known as Pou5f1) transcription by its distal enhancer, retaining a pre-inactivation X chromosome state, and global reduction in DNA methylation and in H3K27me3 repressive chromatin mark deposition on developmental regulatory gene promoters. Upon withdrawal of 2i/LIF, naive mouse ES cells can drift towards a primed pluripotent state resembling that of the post-implantation epiblast. Although human ES cells share several molecular features with naive mouse ES cells, they also share a variety of epigenetic properties with primed murine epiblast stem cells (EpiSCs). These include predominant use of the proximal enhancer element to maintain OCT4 expression, pronounced tendency for X chromosome inactivation in most female human ES cells, increase in DNA methylation and prominent deposition of H3K27me3 and bivalent domain acquisition on lineage regulatory genes. The feasibility of establishing human ground state naive pluripotency in vitro with equivalent molecular and functional features to those characterized in mouse ES cells remains to be defined. Here we establish defined conditions that facilitate the derivation of genetically unmodified human naive pluripotent stem cells from already established primed human ES cells, from somatic cells through induced pluripotent stem (iPS) cell reprogramming or directly from blastocysts. The novel naive pluripotent cells validated herein retain molecular characteristics and functional properties that are highly similar to mouse naive ES cells, and distinct from conventional primed human pluripotent cells. This includes competence in the generation of cross-species chimaeric mouse embryos that underwent organogenesis following microinjection of human naive iPS cells into mouse morulas. Collectively, our findings establish new avenues for regenerative medicine, patient-specific iPS cell disease modelling and the study of early human development in vitro and in vivo.
Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes.[Pubmed:8772178]
FEBS Lett. 1996 Aug 26;392(2):77-80.
Various inhibitors were tested for their potential to suppress the kinase activity of protein kinase C mu (PKC mu) in vitro and in vivo. Among the staurosporine-derived, rather selective PKC inhibitors the indolocarbazole Go 6976 previously shown to inhibit preferentially cPKC isotypes proved to be a potent inhibitor of PKC mu with an IC50 of 20 nM, whereas the bisindolylmaleimide Go 6983 was extremely ineffective in suppressing PKC mu kinase activity with a thousand-fold higher IC50 of 20 microM. Other strong inhibitors of PKC mu were the rather unspecific inhibitors staurosporine and K252a. Contrary to the poor inhibition of PKC mu by Go 6983, this compound was found to suppress in vitro kinase activity of PKC isoenzymes from all three subgroups very effectively with IC50 values from 7 to 60 nM. Thus, Go 6983 was able to differentiate between PKC mu and other PKC isoenzymes being useful for selective determination of PKC mu kinase activity in the presence of other PKC isoenzymes.


